The decades-long, frequently overly complex prepare of bringing a modern medicate to market—a travel full with tall fetched and tall attrition—is experiencing a emotional, constrained advancement. At the heart of this change is the pressing require for quickened clinical trials to quickly provide evidence-based medicines to patients, a need underscored by later worldwide wellbeing emergencies and the ever-present request for cures for unmanageable diseases.
This move guarantees quicker get to to life-saving treatments but presents a complex adjusting act between speed and logical thoroughness. The future of therapeutic investigate pivots on effectively exploring this pressure, utilizing modern innovations whereas maintaining the gold standard of evidence.
Background and Verifiable Context
The present day, organized approach to sedate testing is a generally later innovation. For centuries, medicines were based on recounted prove, perception, and convention. The seeds of cutting edge evidence-based pharmaceutical were sown in the 18th century with point of interest thinks about, such as James Lind’s 1747 controlled trial on scurvy, but it wasn’t until the mid-20th century that the Randomized Controlled Trial (RCT) got to be the broadly acknowledged gold standard for demonstrating a drug’s viability and safety.
The 1962 Kefauver-Harris Corrections in the U.S., a reaction to tragedies like the Thalidomide emergency, formalized the necessity for “significant prove” of adequacy, cementing a multi-phase trial structure. Whereas this meticulousness significantly moved forward understanding security, it moreover brought about in a expensive and time-consuming prepare, frequently taking over a decade and billions of dollars to bring a single sedate from revelation to market.
This pressure driven to the creation of assisted audit programs. The U.S. Nourishment and Medicate Administration’s (FDA) Quickened Endorsement pathway, set up in 1992, permitted for prior medicate endorsement for genuine conditions with neglected therapeutic needs based on a “surrogate endpoint” (a degree sensibly likely to anticipate clinical advantage, like tumor shrinkage) or maybe than holding up for a conclusive “clinical endpoint” (like by and large survival). This component, whereas significant for emergencies like the HIV/AIDS scourge, put an exceptional accentuation on speed.

Current Patterns: The Computerized Acceleration
Today, the drive for speeding up is being turbo-charged by mechanical and methodological development. The COVID-19 widespread served as a pot, illustrating that medicate and immunization advancement timelines might be radically compressed without relinquishing logical integrity.
Key patterns forming the future include:
- Artificial Insights (AI) and Machine Learning (ML): AI is revolutionizing medicate revelation, distinguishing potential medicate candidates, and optimizing clinical trial plan. Prescient analytics can pinpoint the most appropriate trial destinations and persistent socioeconomics, endlessly progressing enrollment, a perpetual bottleneck that causes over 80% of conventional trials to miss their enrollment targets.
- Decentralized Clinical Trials (DCTs): DCTs move trial exercises closer to the understanding, utilizing inaccessible observing, wearable gadgets, and telemedicine. This patient-centric approach increments availability, differing qualities, and maintenance, lessening the require for visit, burdensome location visits and making cooperation simpler for topographically far off or mobility-impaired patients.
- Real-World Prove (RWE) Integration: Information collected exterior of conventional clinical trials—from electronic wellbeing records (EHRs), understanding registries, and computerized wellbeing tools—is progressively being utilized to supplement or indeed stand in for conventional Stage IV post-market ponders. This permits for persistent observing of a drug’s security and viability in a broader, more assorted understanding population.
- Adaptive Trial Plans: These adaptable trial conventions permit analysts to alter the consider in real-time based on collecting data—for case, dropping an ineffectual measurements or altering the randomization ratio—making the prepare more productive, moral, and faster.
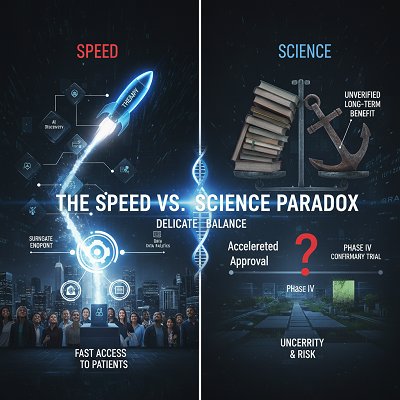
Expert Conclusions: The Speed vs. Science Paradox
The increasing speed of clinical trials has made a separate among specialists with respect to the long-term suggestions for evidence-based treatments.
Proponents contend that development is non-negotiable for open wellbeing. “The moral basic isn’t fair to be secure, but moreover to be quick,” says one industry thought pioneer. “For uncommon maladies or forceful cancers, patients do not have ten a long time to hold up for a conventional Stage III trial. Quickened pathways, when matched with strong post-market observation, are an basic apparatus for giving trust.” They emphasize that innovation minimizes hazard by moving forward information quality and oversight.
Critics, be that as it may, caution that depending intensely on early, surrogate endpoints or smaller-scale thinks about can make an figment of prove. Dr. Joseph Ross, a unmistakable analyst in medicate arrangement, has famous that drugs endorsed through sped up programs are regularly based on shorter and littler trials, driving to a more noteworthy degree of vulnerability upon advertise section. “The concern is what happens when corroborative trials either aren’t completed or fall flat to appear a authoritative clinical advantage,” one open wellbeing teacher famous. This can result in medications of dubious adequacy remaining on the advertise for a long time, driving up healthcare costs and uncovering patients to superfluous dangers. The challenge, specialists concur, is striking the “right adjust” between maximizing get to and guaranteeing the public’s health.
Suggestions for Evidence-Based Treatments
The future of inquire about, characterized by quickened clinical trials, carries significant suggestions for the nature of evidence-based treatments.
Opportunities
- Rapid Reaction to Emergencies: The system built on quickened trials guarantees that the world can react to pandemics and other sudden open wellbeing crises with exceptional speed.
- Precision Medication: Versatile plans and AI-driven information examination encourage the improvement of personalized pharmaceutical, fitting treatments to particular hereditary or atomic profiles, which requires littler, speedier, more focused on trials.
- Increased Get to and Differing qualities: Decentralized models inalienably break down topographical and financial boundaries, driving to trials with more different and agent quiet populaces, which, in turn, reinforces the generalizability of the evidence.
Challenges
- Erosion of Prove Quality: The essential challenge is the hazard of favoring medications with inadequately long-term information. If the required corroborative post-approval (Stage IV) considers are not completed in a opportune manner—a authentic issue tormenting the Quickened Endorsement program—drugs with unverified benefits can stay commercially available.
- Data Judgment and Security: The move to RWE, wearables, and decentralized information collection presents unused complexities with respect to information standardization, protection, and security over worldwide, different systems.
- Regulatory Adjustment: Administrative offices must ceaselessly advance their rules to keep pace with development, making modern systems for approving computerized biomarkers and RWE whereas keeping up open believe in the survey process.
The time of quickened clinical trials marks a definitive turn in restorative investigate. It is a basic step towards a more effective, patient-centric, and eventually, quicker way to cures. In any case, the keenness of evidence-based medication requests that speed remains fastened to logical thoroughness. The future victory of this unused investigate worldview will be measured not fair by how rapidly a medicate comes to the advertise, but by the evident confirmation that it makes a difference individuals live longer, more advantageous lives.





