The pharmaceutical world is experiencing a seismic move, moving from small-molecule pills to large-molecule biologics—complex helpful proteins like monoclonal antibodies (mAbs), cell and quality treatments, and immunizations. These next-generation medications guarantee phenomenal exactness in treating illnesses from cancer to immune system disarranges. Be that as it may, their estimate and complexity show gigantic challenges for examination. Enter Mass Spectrometry (MS), the expository workhorse that is quick getting to be the crucial eye of the biopharma storm.
MS, once a specialty apparatus for small-molecule chemistry, has advanced into a powerhouse for characterizing the exceedingly heterogeneous nature of biologics, driving speedier improvement, guaranteeing administrative compliance, and eventually, shielding persistent health.
A Storied Past Meets a Complex Display: Chronicled Context
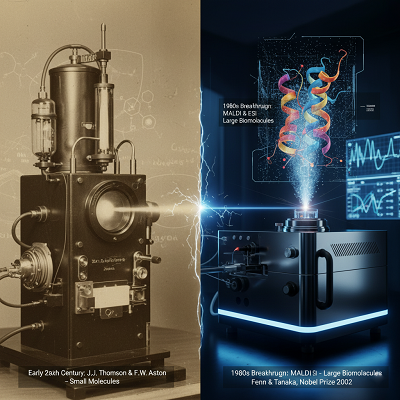
The principal standards of mass spectrometry—measuring the mass-to-charge proportion (m/z) of particles to decide the atomic composition of a sample—were spearheaded in the early 20th century by J.J. Thomson and F.W. Aston. For decades, its utility was to a great extent restricted to characterizing little, unstable particles, serving essentially the oil and chemical industries.
The genuine “coming of age” for biomolecular MS, in any case, arrived in the late 1980s with the advancement of two game-changing ionization strategies: Matrix-Assisted Laser Desorption/Ionization (MALDI) and Electrospray Ionization (ESI). These strategies, which earned John Fenn and Koichi Tanaka a share of the 2002 Nobel Prize in Chemistry, empowered the tender exchange of huge, delicate biomolecules like proteins and peptides into the vaporous, ionized state without dividing them.
This breakthrough coincided impeccably with the rise of the to begin with protein-based therapeutics. All of a sudden, researchers had a apparatus able of affirming the essential amino corrosive grouping of their recombinant proteins. Nowadays, MS has moved distant past basic arrangement affirmation to ended up the de facto standard for in-depth biotherapeutics characterization, supporting each organize from disclosure to quality control.
Current Patterns: A More profound Jump into Biologics Characterization
The complex fabricating prepare of a biologic, frequently including living cell lines, presents various varieties that can influence the drug’s security and viability. These varieties, known as Basic Quality Traits (CQAs), must be fastidiously observed. Mass spectrometry is interestingly situated to handle this complexity.
The CQA Detective
- Post-Translational Alterations (PTMs): Nearly all proteins are altered after interpretation, with changes like glycosylation (the connection of sugar atoms) being especially basic for a therapeutic’s strength and immunogenicity. LC-MS (Fluid Chromatography coupled with Mass Spectrometry) is the gold standard for mapping PTM destinations and deciding the exact composition and plenitude of these adjustments. For occurrence, diverse glycan designs on a monoclonal counter acting agent can definitely change its restorative effect.
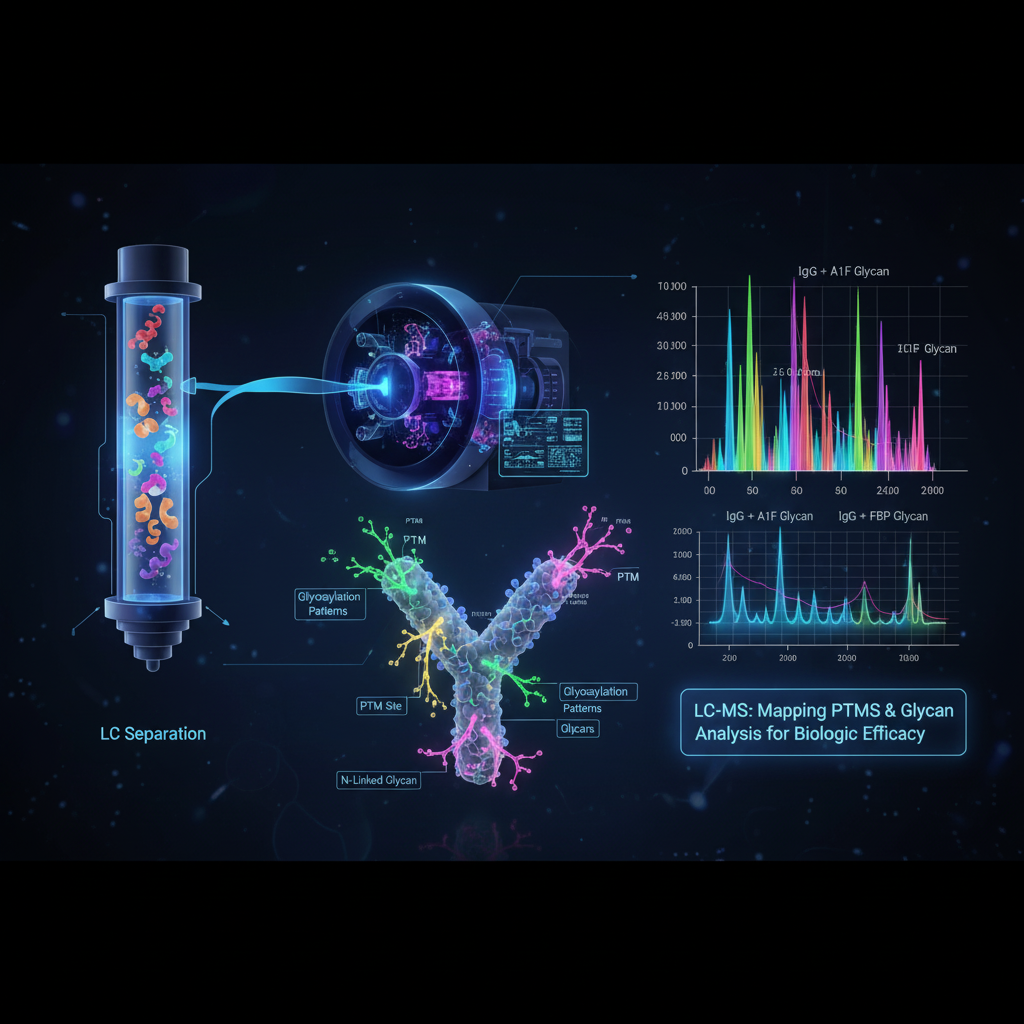
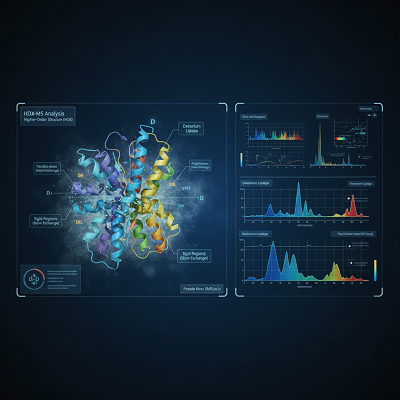
- Higher-Order Structure (HOS): A biologic’s 3D structure—its collapsing and conformation—is key to its work. Procedures like Hydrogen-Deuterium Trade Mass Spectrometry (HDX-MS) are progressively utilized to test this HOS. By observing the trade rate of labile hydrogen particles for deuterium, HDX-MS gives a ‘molecular breathing’ profile, uncovering auxiliary changes at the amino corrosive level with moo test consumption.
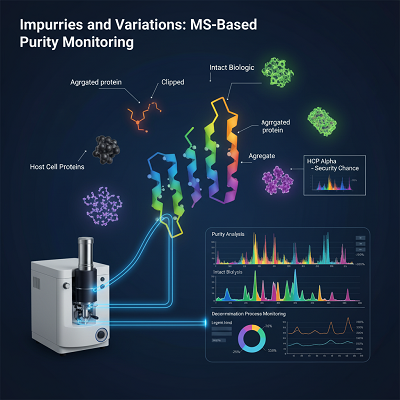
- Impurities and Variations: Biologics are once in a while a single, uniform particle. MS is basic for distinguishing and evaluating product-related variations (e.g., clipped, totaled, or divided proteins) and Have Cell Proteins (HCPs)—residual proteins from the cell line utilized for generation. The nearness of indeed follow sums of tricky HCPs can posture a security chance, and MS-based proteomics permits for their recognizable proof and checking all through the decontamination process.
Accelerating the Pipeline
The coming of High-Resolution, Accurate-Mass (HRAM) MS disobedient (such as Orbitraps and Time-of-Flight (ToF) analyzers) gives the fundamental affectability and settling control to analyze these complex blends rapidly. Moreover, the integration of MS specifically into upstream and downstream manufacturing—often alluded to as “Mass Spec to the Plant”—is changing handle improvement, empowering real-time observing and in-line quality control, which quickens administrative endorsement timelines.
Expert Supposition: The Move to Confidence
“MS is no longer fair a characterization apparatus; it’s a foundational innovation that offers a level of atomic detail and certainty that conventional strategies essentially can’t coordinate,” says Dr. Anya Sharma, a driving biopharmaceutical explanatory chemist. “When you record a Biologics Permit Application (BLA) nowadays, the profundity of MS information required to demonstrate the drug’s basic astuteness and immaculateness is monstrous. It’s the dialect of cutting edge biopharma quality.”
Another developing agreement among industry specialists is the potential for MS to streamline the improvement of biosimilars—generic adaptations of biologics. Exceedingly comparable MS profiles are significant for illustrating “biosimilarity” to the unique trailblazer item, giving a more strong explanatory establishment than was already conceivable with lower-resolution techniques.
Implications for the Future: Personalization and Precision
The part of mass spectrometry is set to grow drastically, not as it were in the improvement lab but moreover in the clinic, impelling the industry toward accuracy medicine.
- Cell and Quality Treatment: As the industry handles indeed more complex therapeutics, such as Adeno-Associated Infection (AAV) vectors for quality treatment, MS is venturing up. Local MS is appearing guarantee as a quick, low-sample-consumption strategy to characterize the intaglio AAV capsid and decide the proportion of “full” (containing the hereditary payload) to “purge” capsids—a vital quality metric.
- Clinical Checking and Pharmacokinetics: High-sensitivity, quantitative MS measures are progressively being created for Helpful Sedate Observing (TDM). This permits clinicians to absolutely degree the concentration of a biologic sedate and its metabolites in a patient’s blood, optimizing person dosing based on a patient’s particular digestion system and reaction, hence maximizing viability and minimizing antagonistic effects.
- Data Science and Mechanization: The future will see MS disobedient getting to be indeed more computerized and user-friendly, pushing them out of specialized center labs and into schedule quality control. This move is intensely dependent on propels in Counterfeit Insights (AI) and Machine Learning (ML) to prepare and decipher the tremendous, complex datasets MS produces, changing crude spectra into noteworthy experiences speedier than ever before.
In quintessence, mass spectrometry is not fair keeping pace with the complexity of biologics; it is effectively forming the improvement of the another era of solutions, guaranteeing that the guarantee of biopharma is conveyed with exceptional precision and safety.





