The U.S. framework of licenses and advertise eliteness is the bedrock upon which the nation’s development economy, especially the effective pharmaceutical industry, is built. It works on a foundational deal: in trade for freely unveiling an innovation, the government awards the innovator a transitory, select imposing business model. This article digs into the complex U.S. viewpoint on these rights, investigating their verifiable roots, the current vital patterns, the wrangle about over their social fetched, and the significant suggestions for development and open access.
Background and Chronicled Foundation
The concept of mental property assurance in the U.S. is revered in the Structure, giving Congress the control “[t]o advance the Advance of Science and valuable Expressions, by securing for constrained Times to Creators and Creators the elite Right to their particular Compositions and Discoveries.”

The Advancement of Exclusivity
- Early Acts (1790, 1836): The to begin with obvious act was passed in 1790, and the cutting edge U.S. obvious framework was generally built up by the Obvious Act of 1836. This act made the U.S. Obvious Office (presently the USPTO) and foundations a formal examination handle, moving absent from a basic enlistment framework. The objective was to guarantee that licenses were allowed as it were for genuine and valuable innovations.
- The 20-Year Term: Nowadays, a utility patent—the most common type—grants the creator an select right for 20 a long time from the date the application was filed.
- The Hatch-Waxman Act (1984): Formally the Sedate Cost Competition and Obvious Term Reclamation Act, this point of interest enactment is central to pharmaceutical restrictiveness. It made the advanced system for bland sedate endorsement, permitting bland producers to record an Truncated Unused Sedate Application (ANDA). Significantly, it moreover presented components for Obvious Term Rebuilding (PTR) to compensate medicate trailblazers for time misplaced amid the long FDA administrative endorsement handle, as well as a few shapes of administrative restrictiveness allowed by the FDA that run concurrently with patents.

A Layered Defense: Licenses vs. Administrative Exclusivity
For unused drugs, advertise assurance is regularly a combination of obvious rights and administrative exclusivities, making a layered defense against nonexclusive competition.
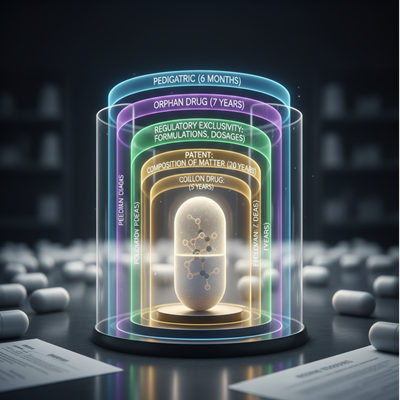
Patents: The Center Right
The most vital obvious is regularly the composition of matter obvious, which ensures the dynamic pharmaceutical fixing (API) itself. Pharmaceutical companies moreover record “follow-on” licenses that cover unused details, strategies of utilize (e.g., treating a unused illness), or particular dosages.
The Viable Obvious Life Challenge: Whereas the ostensible obvious term is 20 a long time, the R&D and clinical trial handle for a modern medicate frequently devours 7 to 12 a long time of that time. As a result, the successful obvious life—the real period of advertise restrictiveness delighted in after FDA approval—is regularly much shorter, ordinarily averaging 11 to 14 a long time for top-selling drugs.
Regulatory Exclusivities: Time-Limited Protection
The FDA awards particular periods of advertise restrictiveness, in any case of obvious status, to incentivize certain sorts of R&D:
- New Chemical Substance (NCE) Restrictiveness: Five a long time of eliteness for a medicate containing an dynamic fixing never some time recently approved.
- Orphan Sedate Eliteness (Tribute): Seven a long time of eliteness for drugs treating uncommon maladies (influencing less than 200,000 individuals in the U.S.).
- Pediatric Eliteness: An extra six months included to existing licenses or exclusivities for conducting ponders on the sedate in pediatric populations.
Current Patterns and Vital Maneuvers
The current obvious scene is characterized by high-stakes case, vital obvious portfolio administration, and seriously open examination over medicate pricing.
The Rise of “Obvious Shrubberies” and “Evergreening”

One of the most questionable patterns is the utilize of obvious thickets—accumulating handfuls of auxiliary licenses around a single medicate to make a cautious barrier.
- Evergreening: This procedure includes recording afterward licenses for minor changes, unused details, or diverse strategies of utilize for an existing medicate. Pundits contend this hone falsely amplifies showcase eliteness well past the life expectancy of the unique composition of matter obvious, a hone they call “evergreening.”
- The Money related Affect: Whereas the USPTO and a few considers fight that the number of licenses on a medicate doesn’t relate straightforwardly with bland passage timing, other inquire about recommends that these auxiliary licenses collectively delay non specific competition by an normal of 2 to 3 a long time, costing shoppers and payers billions of dollars annually.
The Associate Partes Audit (IPR) Challenge
The America Designs Act (AIA) of 2011 presented the Associate Partes Audit (IPR) handle, which permits third parties to challenge the legitimacy of an issued obvious at the USPTO’s Obvious Trial and Request Board (PTAB) on grounds of oddity or obviousness.
- Generic Methodology: IPR has gotten to be a capable, generally speedier, and cheaper device for nonexclusive producers to challenge the legitimacy of brand-name medicate licenses, frequently concurrently with the more conventional area court litigation.
The Biosimilars Framework
For biologics (complex drugs like insulins, antibodies, and immunizations), the U.S. made a particular restrictiveness pathway.
- BPCIA Restrictiveness: The Biologics Cost Competition and Development Act (BPCIA) gifts originators 12 a long time of advertise restrictiveness for a modern biologic, a longer period than for conventional small-molecule drugs, due to the higher complexity and fetched of R&D. This system is vital to the rising showcase of biosimilars, which are comparable, cheaper adaptations of biologics.
Expert Suppositions and The Development Debate
The talk about over licenses and restrictiveness bubbles down to a principal address of adjust: incentivizing development versus advancing open get to and affordability.

The Innovator’s Viewpoint (Motivating force Theory)
Pharmaceutical officials and industry advocates contend that solid, enforceable obvious rights are existential.
“The guarantee of eliteness is the motor that drives biomedical development. Without the capacity to recover the billions of dollars went through on R&D—where 90% of all candidates fail—there would be no capital to seek after the another era of life-saving drugs. Debilitating obvious assurance is a coordinate danger to future cures.”
The Critic’s Point of view (Get to and Affordability)
Consumer advocates, nonexclusive producers, and certain policymakers contend that current obvious hones have tipped the adjust as well distant toward monopoly.
“When a medicate loses obvious security, costs fall by 80-90% nearly quickly. The vital layering of follow-on licenses is a frame of administrative mishandle planned to draw out imposing business model estimating, not to drive genuine helpful development. The social taken a toll of these postponed non specific passages is a major open wellbeing crisis.”
Implications for the Future
The pressures inside the U.S. obvious and restrictiveness framework have far-reaching implications.
The ‘Patent Cliff’ and R&D Focus
The approaching ‘patent cliff’—the period where a huge number of blockbuster drugs will lose exclusivity—is constraining brand-name companies to move their R&D center toward complex, specialized regions like cell and quality treatments and oncology, where higher costs legitimize the high-risk venture. This move may result in less “mass-market” medicate innovations.
Legislative and Administrative Scrutiny
Policymakers proceed to look for ways to check seen mishandle of the obvious framework without wrecking R&D motivations. Later activities by the Federal Trade Commission (FTC) flag an expanding eagerness to scrutinize the posting of auxiliary licenses in the FDA’s Orange Book (the open list of endorsed sedate items with obvious and restrictiveness data) for signs of out of line competition. Potential administrative changes regularly rotate around restricting the scope or number of follow-on licenses that can be stated in litigation.
The Worldwide Standard
As the world’s driving center for biomedical R&D, the U.S. obvious system acts as a de facto worldwide standard. Changes to the U.S. framework, driven by the strife between patent-protected sedate estimating and requests for widespread get to, unavoidably swell outward, affecting mental property talks about and healthcare reasonableness around the world. The future of U.S. restrictiveness rights will be characterized by its capacity to strike a feasible, moral adjust between fulfilling the creator and serving the open good.





