Pharmaceutical bundling is distant more than a basic holder; it is the final line of defense ensuring a product’s judgment, guaranteeing administrative compliance, and eventually defending quiet wellbeing. In the exceedingly competitive and thoroughly directed worldwide healthcare scene, guaranteeing productivity in the bundling handle is not simply a cost-saving degree but a key, operational, and moral basic for pharma bundling companies.
Background: The Double Part of Pharmaceutical Packaging
Historically, the essential work of pharmaceutical bundling was crucial assurance against natural components like dampness, light, and defilement. In old and medieval times, this included utilizing normal materials like clay, glass, and creature skins. The Mechanical Transformation brought mass generation, standardizing materials like glass vials and, afterward, the development of the rankle pack, which revolutionized unit-dose bundling and persistent adherence.
The advanced time, be that as it may, saw bundling advance into a complex framework with double, non-negotiable roles:
- Item Keenness and Security: Keeping up sedate solidness, avoiding corruption, and guaranteeing sterility.
- Data and Compliance: Giving clear measurement enlightening, notices, and the ordered serialization information for traceability and anti-counterfeiting efforts.
This complexity, coupled with zero resistance for mistake, is absolutely why productivity is vital. An productive prepare is an exact, compliant, and cost-effective handle, specifically affecting the time-to-market and the accessibility of life-saving medicines.
The Current Scene: A Three-Pillar Challenge
Today’s pharmaceutical bundling companies work beneath seriously weight driven by administrative requests, showcase elements, and natural duty. Effectiveness is the key to overseeing this complexity.
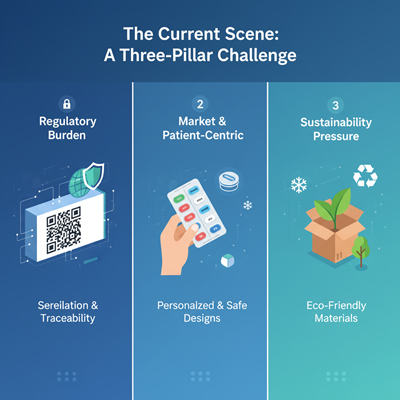
- The Administrative Burden (Security & Traceability)
Regulatory bodies like the FDA (U.S.) and EMA (Europe) force rigid cGMP (current Great Fabricating Hones) and serialization prerequisites. Each unit must have a one of a kind identifier (a 2D standardized identification or QR code) permitting for track-and-trace all through the worldwide supply chain. Wasteful, manual, or ineffectively coordinates bundling lines risk:
- Mislabeling and Serialization Mistakes: These are basic abandons that can lead to exorbitant, life-threatening recalls.
- Compliance Downtime: Non-validated or moderate forms lead to administrative reviews, generation solidifies, and enormous money related penalties.
2. Showcase and Patient-Centric Demands
The showcase presently requires bundling to be more complex, personalized, and helpful. This includes:
- Child-Resistant & Senior-Friendly Plans: Requiring two-step opening components that must be absolutely executed on the line.
- Patient-Specific Bundling: Catering to custom fitted doses or complex regimens (e.g., week by week rankle cards).
- Temperature-Sensitive Drugs: The rise of biologics and immunizations requires proficient cold chain bundling, requesting specialized, approved arrangements that keep up steady temperatures.
3. Supportability Pressure
The industry faces expanding weight to receive ecologically neighborly hones. Proficiency here implies fabric optimization. Companies are moving toward:
- Mono-material bundling (utilizing a single sort of recyclable polymer) to rearrange recycling.
- Moderate plan to decrease in general fabric consumption.
- Biodegradable and plant-based plastics for rankle packs.
These moves require bundling lines to proficiently handle unused, frequently less-rigid materials without compromising high-barrier assurance, a critical designing challenge that requests optimized processes.
Current Patterns: Innovation Driving Efficiency
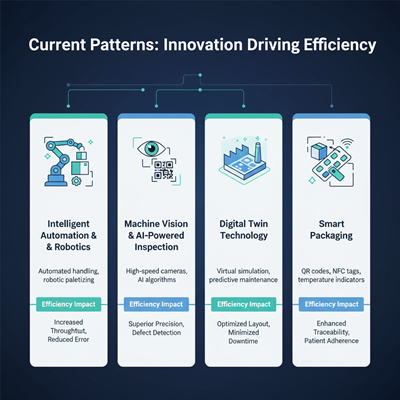
The drive for proficiency is fueling quick mechanical advancement over the bundling line:
| Trend | Description | Efficiency Impact |
| Intelligent Robotization & Robotics | Automated taking care of of dreary errands like tallying, filling, and fixing, coupled with mechanical palletizing. | Increased Throughput, Decreased Human Mistake (the single most prominent chance figure), Reliable Quality. |
| Machine Vision and AI-Powered Inspection | High-speed cameras and fake insights calculations check names, seals, and items in real-time. | Superior Precision, Real-Time Deformity Location, minimizing downstream squander and rework. |
| Digital Twin Technology | Virtual recreation of the whole bundling line some time recently physical production. | Optimized Line Format, Minimized Approval Time, and Prescient Support to dispose of unforeseen downtime. |
| Smart Packaging | Integration of advanced apparatuses like QR codes, NFC labels, and time-temperature markers (TTIs) into the bundling itself. | Enhanced Traceability, Progressed Stock Administration (RFID), and Superior Quiet Adherence. |
Expert Conclusions: The Taken a toll of Inefficiency
Industry specialists generally concur that a disappointment to prioritize bundling proficiency has serious money related and open wellbeing implications.
Shannon Walker, Vice-President of Quality for a major bundling arrangements firm, has already famous that the most prominent dangers are not in the machine but in the process—particularly dangers related with human variables. “Computerization controls the dangers related with human application,” lessening the chance of basic print surrenders or item mix-ups that seem alter the meaning of doses, driving to “basic results for the patient.”
Consultants specializing in supply chain optimization emphasize the financial aftermath. “In a profoundly directed environment, wastefulness breaks even with hazard, and hazard breaks even with taken a toll,” says one industry ingenious. Wasteful lines are characterized by:
- Intemperate Downtime: Spontaneous support or visit changeovers slice operational utilization.
- Tall Squander: Ineffectively calibrated apparatus leads to fabric abuse or defective bundling that must be scrapped.
- Expanded Revamp: Blunders in labeling or fixing require costly, time-consuming reprocessing.
These covered up costs expand the add up to fetched of merchandise sold, dissolve benefit edges, and, fundamentally, can cause supply chain disturbances, debilitating the consistent supply of imperative solutions to patients around the world.
Suggestions: Securing the Future

Ensuring bundling prepare effectiveness is the single most successful way for pharmaceutical companies to de-risk their operations and secure future growth.
For patients, proficiency implies lower chance of mislabeled or compromised pharmaceutical, superior openness, and progressed adherence through user-friendly, well-designed packaging.
For companies, it interprets straightforwardly into operational fabulousness: quicker time-to-market for modern drugs, a diminished natural impression, and the adaptability required to handle assorted item portfolios and fluctuating worldwide demand.
The way forward requires vital venture in the most recent mechanization advances, a commitment to data-driven prepare mapping for persistent enhancement, and a culture that sees the bundling line as a mission-critical component of open wellbeing, not just the conclusion of the generation handle. The bundling prepare effectiveness motivation is a capable union of security, compliance, and benefit.





