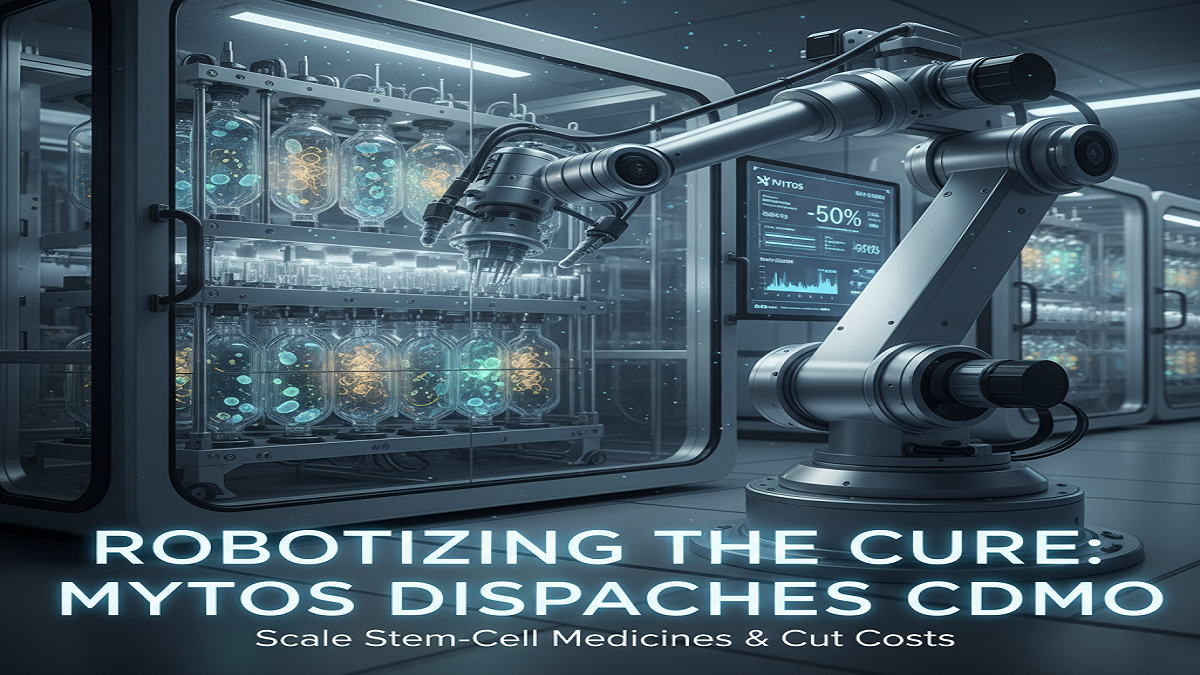
The ensure of regenerative medicine—using living cells to repair hurt tissue—has long been hampered by a essential, industrial-scale bottleneck: Manufacturing. The get ready of creating and isolating billions of stem cells is costly, exceedingly manual, and troublesome to standardize.
In a move sketched out to break that bottleneck, biotechnology company Mytos has announced the celerity of its Automated Contract Change and Creating Organization (CDMO). Fueled by its prohibitive iDEM™ computerization development, the present day CDMO illustrate focuses to pass on versatile, sensible, and high-quality era of stem cell-derived medications, in a common sense changing the money related things of cell and quality medicine.
Background: The Obliterating Taken a toll of Manual Cell Culture
Stem cell-derived medicines, which consolidate everything from activated pluripotent stem cells (iPSCs) for Parkinson’s ailment to begetter cells for bone repair, talk to the cutting edge of pharmaceutical. In any case, their way to wide calm get to is blocked by creating challenges:
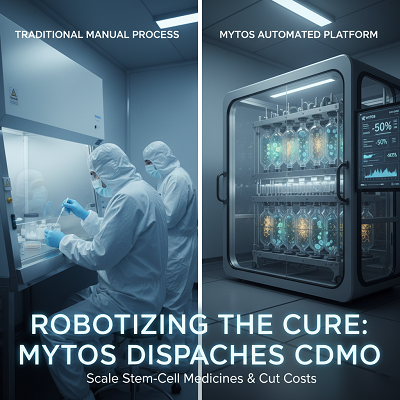
- Labor-Intensive: Generally, creating and managing with cells is done physically in cleanrooms by exceedingly arranged experts. This makes the handle direct, exorbitant, and subordinate on uncommon human expertise.
- Cost and Scale: The reliance on manual shapes suggests that labor accounts for a vital divide of the include up to era gotten, driving to final treatment costs that can be prohibitively tall. Scaling up requires building more expensive cleanrooms and enrolling more staff, a handle that is direct and capital-intensive.
- Variability and Debasement: Human intercession increases the chance of batch-to-batch changeability and microbial debasement, which can lead to extreme bunch dissatisfactions and regulatory delays.
The iDEM™ Solution
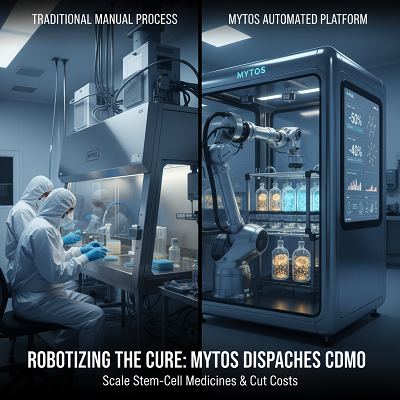
Mytos addresses these issues with its iDEM™ mechanization organize. The development mechanizes all the labor-intensive unit operations in cell culture—such as coating, seeding, feeding, imaging, and harvesting—while keeping up compatibility with the same 2D T-flask organize utilized in manual culture.
This “no-change” approach is key since it licenses biotech companies to rapidly trade their existing manual traditions to a closed, computerized system without having to re-engineer the cell science, through and through enlivening the way to clinical readiness.
Current Designs and Mytos’s Strategy
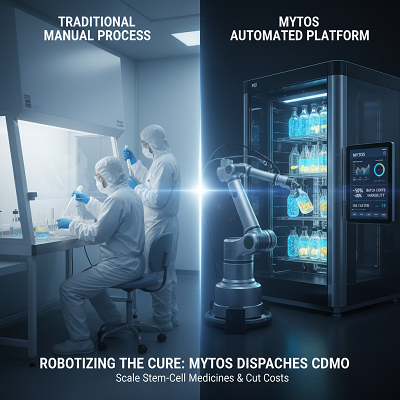
Mytos’s celerity is eminently arranged with the megatrend of mechanization clearing the cell and quality treatment (CGT) CDMO promote. The around the world CGT CDMO publicize is expected to create radically over the taking after decade as more medicines enter late-stage clinical trials.
The Regard Proposition
Mytos is arranging its computerized CDMO as the course of action for both scale-out (more patient-specific estimations) and brought reduction.
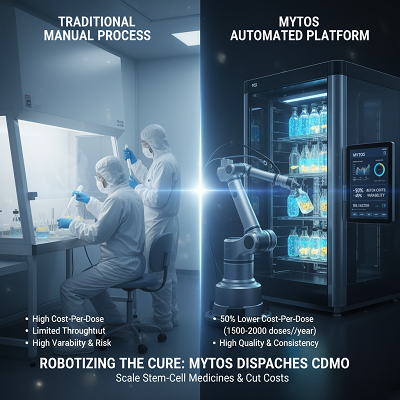
- Cost-Per-Dose Diminishment: Mytos claims the computerized system can fulfill up to 50% lower clump costs compared to manual CDMOs, essentially by lessening the labor and cleanroom impression required by up to tenfold.
- Throughput: The to start with office, housed interior the Cell and Quality Treatment Catapult’s (CGT Catapult) Stevenage Creating Progression Center in the UK, is centering on an annually era of up to 1,500 autologous (patient-specific) or 25,000 allogeneic (off-the-shelf) doses.
- Accessibility: The CDMO appear grants treatment engineers to get to Extraordinary Manufacturing Sharpen (GMP)-ready capacity rapidly, bypassing the require for huge blunt capital wanders and long equipment endorsement timelines.
Mytos’s collaborations with biotech companies like Aspen Neuroscience (Parkinson’s illness), StemSight (corneal visual impedance), and Rinri Therapeutics (hearing mishap) outline the platform’s adaptability over diverse cell sorts and regenerative pharmaceutical applications.
Expert Supposition and Future Implications
Industry veterans are hailing this turn toward computerized CDMOs as fundamental for the commercial reasonability of regenerative medicine.
Dr. David DiGiusto, a cell and quality treatment manufacturing advisor, communicated that Mytos’s approach has the “potential to characterize manufacturing benchmarks” by passing on relentless, low-cost clumps at the scale required to reach patients globally.
The recommendations of viable robotized CDMO scaling are profound:
- Mass Understanding Get to and Affordability
By radically bringing down the Taken a toll of Items (COG) for stem cell medications, Mytos and comparable computerized stages may make current high-cost drugs accessible to a much greater understanding masses. The center shifts from clinical plausibility to commercial scalability.
- Democratization of Cell Treatment Development
Small and medium-sized biotechs, which routinely require the huge capital to build their have GMP workplaces, can directly outsource manufacturing to an robotized, high-throughput accessory. This brings down the hindrance to segment for creative steady engineers, conceivably animating the entire field.
- A Unused Standard for Quality and Consistency
Replacing variable manual labor with mechanical innovation and sensors ensures a higher degree of batch-to-batch consistency, a key need for regulatory bodies like the FDA and EMA. This should to translate to less bunch dissatisfactions and a faster way through regulatory approval.
While the Stevenage office is the to start with step, Mytos has communicated plans for a orchestrate of around the world regions. If this organize succeeds in illustrating the monetary illustrate, the mechanized CDMO may development from a claim to fame course of action to the fundamental manufacturing spine of the entire regenerative medicine industry. The time of manual cell culture is rapidly giving way to an mechanical guerilla sketched out to make cures both versatile and sensible.