A modern course of therapeutics, Antibody-Oligonucleotide Conjugates (AOCs), is developing from the meeting of two particular biomedical areas: the focused on precision of monoclonal antibodies (mAbs) and the gene-modulating control of oligonucleotide treatments. Propelled by the clinical victory of Antibody-Drug Conjugates (ADCs), AOCs are planned to overcome the basic jump of systemic conveyance that has long constrained the potential of RNA-based medications. Whereas no AOC has however secured last FDA endorsement, the most recent clinical information and fast headways in overcoming complex chemical and specialized challenges show this innovation is balanced to revolutionize the treatment of complex illnesses, especially those influencing tissues past the liver.
Background and Chronicled Setting: The Point of reference of Conjugation
The history of AOCs is established in the improvement of focused on bioconjugates. Monoclonal Antibodies (mAbs), to begin with created in the 1970s, advertised the capacity to specifically tie to antigens on the surface of particular cells, like cancer cells. This focusing on control driven to the concept of the Antibody-Drug Conjugate (ADC) in the early 2000s, where a cytotoxic little particle “payload” is chemically connected to an counter acting agent. The counter acting agent conveys the sedate specifically to the target cell, minimizing systemic introduction and decreasing the extreme side impacts of conventional chemotherapy.
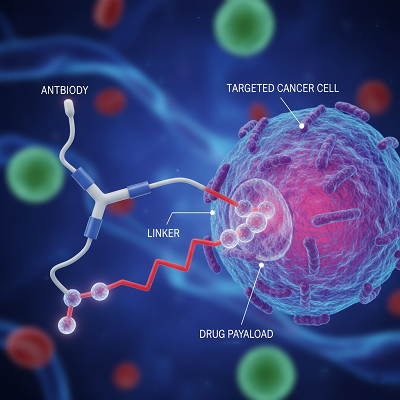
The victory of ADCs—with over a dozen endorsed for oncology indications—set the organize for AOCs. Not at all like ADCs, which utilize a little particle to murder the cell (cytotoxic payload), AOCs utilize an oligonucleotide (such as a little interferometer RNA, or siRNA, or an antisense oligonucleotide, or ASO) as their payload. This oligonucleotide payload is outlined to intercede at the hereditary level, either quieting a destructive quality or rectifying a flawed protein-coding message.
The Oligonucleotide Conveyance Problem
Historically, oligonucleotide treatments themselves have battled with conveyance. Being expansive, contrarily charged particles, they are quickly cleared from the circulatory system, are vulnerable to enzymatic debasement, and have trouble entering cell films, particularly in tissues exterior the liver. Whereas a few non-liver focusing on procedures exist, such as LNP (Lipid Nanoparticle) conveyance, they need the cellular exactness advertised by an antibody.
AOCs straightforwardly address this restriction. The counter acting agent component ties to a particular cell-surface receptor—such as Transferrin Receptor 1 (TfR1) for muscle-targeting—and encourages receptor-mediated endocytosis, internalizing the whole conjugate. Once interior the target cell, the oligonucleotide is discharged to apply its gene-modulating impact. This is the center quality: utilizing the counter acting agent to open extrahepatic (non-liver) conveyance for oligonucleotide therapeutics.
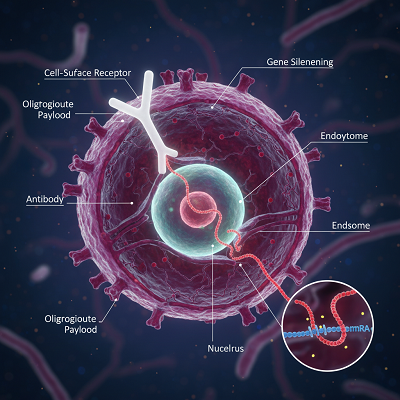
Overcoming Imposing Specialized and Chemical Hurdles
The travel from concept to clinical reality for AOCs is a confirmation to advanced chemical designing, as conjugating two complex biomolecules—a ≈150 kDa counter acting agent and a ≈10 kDa oligonucleotide—presents one of a kind challenges:
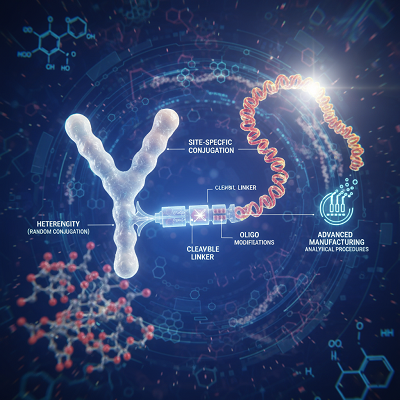
| Challenge | Description | Innovation/Solution |
| Heterogeneity | Random conjugation (e.g., to lysine buildups) leads to a blend of items with shifting oligonucleotide-to-antibody proportions (Paddle), affecting solidness and function. | Site-Specific Conjugation: Designed antibodies (e.g., utilizing THIOMAB innovation or unnatural amino acids) or “tap chemistry” guarantees a characterized, steady Paddle and conjugation location, driving to a more homogeneous and reproducible medicate product. |
| Linker Steadiness & Release | The chemical “linker” must be steady in the circulation system to anticipate untimely payload discharge but cleavable interior the target cell to permit the oligonucleotide to function. | Advanced Linker Chemistries: Improvement of cleavable linkers responsive to the intracellular environment (e.g., moo pH or particular proteins) and non-cleavable linkers that depend on lysosomal debasement for controlled release. |
| Oligonucleotide Integrity | The oligonucleotide payload must be chemically adjusted to stand up to debasement by nucleases and keep up its capacity to tie to its target RNA. | Oligo Adjustments: Utilize of chemistries like Phosphorothioate (PS) spines and 2′-sugar adjustments (e.g., 2′-O-methyl) to upgrade soundness and target affinity. |
| Manufacturing and Analytics | Scaling generation and guaranteeing batch-to-batch consistency for a half breed biologic of this estimate and complexity. | Advanced Explanatory Procedures: Utilize of high-resolution mass spectrometry, capillary electrophoresis, and ion-exchange chromatography to absolutely characterize the Paddle, immaculateness, and conjugation site. |
Manufacturing and Analytics
Scaling generation and guaranteeing batch-to-batch consistency for a half breed biologic of this estimate and complexity.
Advanced Explanatory Procedures: Utilize of high-resolution mass spectrometry, capillary electrophoresis, and ion-exchange chromatography to absolutely characterize the Paddle, immaculateness, and conjugation site.
Current Patterns and Master Opinions
The field has seen a sensational move from scholarly interest to a clinical improvement center, to a great extent driven by companies specializing in this platform.
Leading the Clinical Pipeline
The company Eagerness Biosciences is a clear frontrunner, giving compelling clinical approval for the AOC stage. Their lead candidate, AOC 1001, focusing on the Transferrin Receptor 1 (TfR1) to provide siRNA against DMPK mRNA, has appeared promising comes about in treating Myotonic Dystrophy Sort 1 (DM1). The treatment has gotten Quick Track and Breakthrough Treatment Assignments from the FDA and has progressed to Stage 3 trials, stamping a basic minute for the whole class.

Other key players, such as Dyne Therapeutics (centering on Duchenne Strong Dystrophy and FSHD) and Denali Therapeutics (centered on CNS conveyance), are moreover illustrating the platform’s flexibility past muscle tissue, counting the potential to cross the challenging Blood-Brain Obstruction (BBB).
Expert Outlook
Experts in bioconjugation and RNA therapeutics emphasize the paradigm-shifting nature of AOCs. “The AOC stage is seemingly the to begin with vigorous, generalizable procedure for extrahepatic conveyance of RNA therapeutics,” notes a driving pharmaceutical expert in the field.
The common agreement is that AOCs are not just a alteration of ADCs but a unused helpful lesson through and through, advertising unmistakable preferences for persistent, non-cytotoxic treatments. The capacity to “medicate the undruggable”—targeting the root hereditary cause of maladies in already blocked off tissues—is the central topic of energy among analysts. The current patterns show a pipeline intensely centered on:
- Rare Muscle Infections: DM1, FSHD, DMD.
- Neurological Clutters: Utilizing antibodies that lock in receptors for BBB transcytosis.
- Oncology: Focused on quality quieting in tumors without the systemic poisonous quality of cytotoxic ADCs.
Implications for Patients and the Administrative Landscape
- Patient Implications
The fruitful endorsement of the to begin with AOC would be a momentous move for patients with extreme, as of now unmanageable illnesses. For conditions like Myotonic Dystrophy Sort 1, which influences quality of life and life expectancy with no current healing alternatives, AOCs offer the potential for disease-modifying treatment by means of a moderately occasional systemic infusion. This seem interpret to made strides engine work, decreased infection seriousness, and a superior quality of life.
- Regulatory Challenges
For administrative bodies like the FDA and EMA, AOCs display a one of a kind survey challenge. They are half breed biologics, requesting mastery in both expansive particle (counter acting agent) and nucleic corrosive (oligonucleotide) fabricating, quality control, and clinical security profiles.
“Regulators must evaluate the solidness of the conjugate, the consistency of the Paddle, and the potential for immunogenicity against both the counter acting agent and the oligonucleotide components,” clarifies a previous FDA official. The thoroughness of their expository characterization and control over the conjugation handle will be the extreme determinant of administrative victory. As the to begin with AOCs approach accommodation, the industry will be observing closely to see how administrative benchmarks are formally built up for this cutting-edge helpful class.
In conclusion, the chemical and specialized obstacles for Antibody-Oligonucleotide Conjugates are quickly being cleared by advancement in site-specific conjugation and linker chemistry. This stage is presently moving from preclinical guarantee to late-stage clinical reality, holding the potential to ended up the “third wave” of focused on biopharmaceuticals, conveying the control of quality balance to a wide run of maladies as of now missing viable treatment.





