The completion of a Stage III clinical trial marks a basic intonation point for any pharmaceutical item. Whereas administrative endorsement is the prompt objective, a drug’s genuine impact—its commercial victory, long-term security, and real-world advantage to patients—is decided by a complex set of procedures executed in the post-trial stage, regularly named Stage IV or post-marketing. This arrange is no longer a unimportant administrative convention but a advanced, data-driven space that reshapes the pharmaceutical landscape.
Background and Authentic Setting: From Mystery to Transparency
Historically, the post-trial stage was characterized by a constrained, responsive approach centered fundamentally on required pharmacovigilance—collecting reports of unfavorable occasions (side impacts) once a medicate was on the showcase. Clinical trial information, indeed for fizzled or completed considers, was to a great extent kept mystery by firms, seen as exclusive data earned through expensive research.
A seismic move started in the mid-2000s, initiated by expanding open and administrative requests for straightforwardness and moral responsibility. A key turning point was the US FDA’s move in 2007 requiring the full divulgence of clinical trial information, counting those with negative results. This change, as scholastic investigate recommends, has significantly changed development methodologies, moving the essential driver from unadulterated competition to learning from peers’ triumphs and failures.
Simultaneously, the moral talk about around Post-Trial Get to (PTA) for consider members who profited from the investigational sedate picked up conspicuousness. Whereas the Statement of Helsinki presented PTA as an moral thought in 1964, it is still not generally required, driving to continuous endeavors to build up standardized, feasible instruments for proceeded treatment, especially for patients with uncommon diseases.
Current Patterns: The Rise of Real-World Prove and Quiet Centricity
Today’s post-trial scene is characterized by two major, interlinked patterns: the key arrangement of Real-World Prove (RWE) and an heightens center on Patient-Centricity.
Real-World Prove (RWE)
The cutting edge approach to post-marketing reconnaissance (PMS) has advanced from receptive detailing to proactive, nonstop security and viability observing fueled by RWE.
- Beyond the Trial: RWE envelops information produced from schedule healthcare conveyance, counting Electronic Wellbeing Records (EHRs), claims databases, quiet registries, and advanced wellbeing innovations like wearable gadgets. Clinical trials, by plan, include a little, exceedingly chosen persistent populace beneath controlled conditions. RWE fills a basic crevice by appearing how a medicate performs over different, heterogeneous populaces in the genuine world.
- Regulatory Selection: Administrative bodies, such as the FDA (through its Sentinel Activity) and the EMA, are progressively depending on RWE. This information is utilized to identify uncommon antagonistic occasions not obvious in littler trials, distinguish modern chance variables, back name upgrades, and screen long-term security, a handle regularly named the genuine Stage IV.
- Advanced Analytics: Counterfeit Insights (AI) and Machine Learning (ML) are presently fundamental instruments in RWE era, empowering complex examination of tremendous, assorted datasets to quickly distinguish unpretentious security signals that conventional strategies might miss.
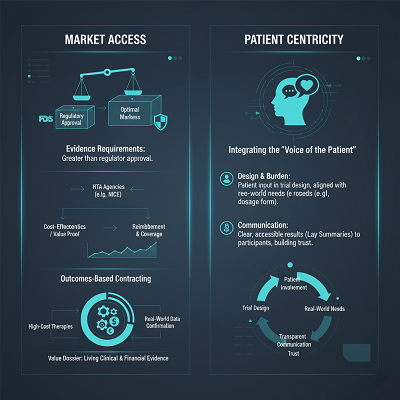
Market Get to and Wellbeing Innovation Appraisal (HTA)
RWE is basic not fair for security but for Advertise Access—the capacity to secure repayment and model inclusion.
- Evidence Necessities: As specialists point out, the evidentiary prerequisites for ideal advertise get to are frequently more noteworthy than those required for administrative endorsement. Payers and HTA offices (like Pleasant in the UK) request prove of a drug’s value—its cost-effectiveness compared to existing treatments. This frequently requires long-term, real-world information to illustrate strong adequacy and taken a toll savings.
- Outcomes-Based Contracting: A rising slant, particularly for high-cost treatments like quality and cell treatments, is outcomes-based contracting. Payers concur to pay for the medicate as it were if real-world information affirms the understanding is accomplishing the guaranteed helpful result, viably joining post-market execution specifically into the monetary model.
Patient Centricity
Pharmaceutical companies are moving toward joining the “Voice of the Understanding” into each stage, counting post-trial.
- Design and Burden: More noteworthy persistent association in trial plan points to minimize understanding burden and guarantee the last item properties (e.g., measurement shape, organization) adjust with real-world understanding needs.
- Communication: Post-trial communication presently amplifies past logical distribution to giving clear, available comes about (lay rundowns) to members and the open, building believe and satisfying an moral responsibility.
Expert Conclusions and Implications
The integration of RWE and advertise get to contemplations early in the advancement pipeline is no longer discretionary; it is a vital imperative.
Implications for Commercialization
Expert agreement recommends that showcase get to challenges, not administrative disappointments, account for the larger part of medicate dispatch failures.
- Early Arranging: “Companies regularly come up short to realize that the evidentiary necessities required for ideal advertise get to are more noteworthy than that required for administrative endorsement,” states one industry preliminary. Effective commercialization requires consolidating payer and HTA viewpoints into early clinical improvement to guarantee the right information is collected from the start.
- The Esteem File: The post-trial stage includes ceaselessly building a energetic esteem dossier—a living report of clinical and financial evidence—that legitimizes the drug’s cost and long-term utilize to diverse partners worldwide.
Implications for Understanding Care
The advancement of post-trial methodologies has significant suggestions for quiet security and ethics.
- Enhanced Security: Proactive RWE-based observation essentially improves persistent security by identifying uncommon, long-term, or subpopulation-specific side impacts distant prior than conventional strategies. The verifiable withdrawal of drugs like Rofecoxib due to cardiovascular hazard, a long time after starting endorsement, serves as a stark case of the basic require for nonstop, advanced monitoring.
- Ethical Commitment: The moral basis for post-trial obligation presently centers on dodging the abuse of members. Giving Post-Trial Get to (PTA) to advantageous mediations is seen as an act of correspondence and an basic portion of the social contract between analysts and the communities that empower their work.

In conclusion, the post-trial stage has changed from a calm, compliance-focused period into a energetic, data-intensive field. By leveraging RWE, grasping patient-centric models, and deliberately joining showcase get to needs, the pharmaceutical industry is moving toward a more straightforward, viable, and morally grounded approach to bringing life-saving medications from the lab seat to the understanding bedside.





