In the fiercely regulated world of pharmaceutical manufacturing, where patient safety is the non-negotiable bottom line, the quest for robust quality assurance is perpetual. While traditional methods like metal detection and vision systems remain vital, a new-age gatekeeper—X-ray inspection technology—is stepping into the spotlight, proving to be a highly viable, multi-functional, and increasingly essential solution for ensuring the integrity of medicines and medical devices.
Background & Historical Context: From Diagnostics to Defense

The application of X-ray technology has a long, illustrious history, dating back to Wilhelm Conrad Röntgen’s discovery in 1895. For decades, its primary use was in medical diagnostics and, later, in non-destructive testing (NDT) across various industries. However, its widespread adoption in pharmaceutical quality assurance (QA) is a more recent trend.
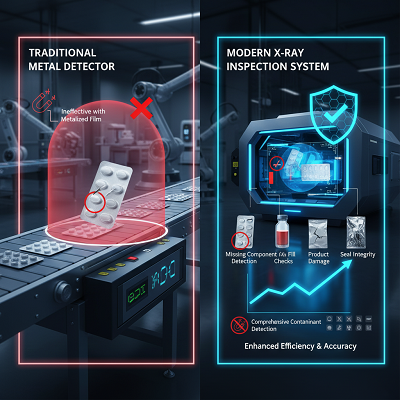
Historically, pharmaceutical manufacturers relied heavily on metal detectors to catch metallic foreign objects. Yet, as packaging became more complex—often involving aluminum foil-based blister packs and metallized films—metal detectors faced limitations. This gap, along with the growing need for internal product and packaging integrity checks, paved the way for the pharmaceutical sector to embrace X-ray systems.
Early industry hesitation was rooted in concerns about radiation exposure potentially compromising drug efficacy. However, extensive research and regulatory guidance, notably from bodies like the US Food and Drug Administration (FDA), have largely allayed these fears. Studies have consistently shown that the extremely low-dose, short-duration X-rays used in inspection systems impart a dose lower than the natural background radiation a product receives in a single day, with no measurable impact on drug quality, dissolution, or hardness of the pharmaceutical products tested. This scientific validation has cemented X-ray inspection’s position as a safe, effective QA tool.
A Multi-Faceted Quality Shield
The viability of X-ray inspection for pharma stems from its versatility and superior detection capabilities compared to single-function inspection equipment:
- Comprehensive Contaminant Detection: Unlike metal detectors, X-ray systems can detect a wide spectrum of high-density foreign bodies, including metal, glass, mineral stone, dense plastics, and rubber, regardless of their position within the product or its packaging material. This is crucial for products packaged in aluminum foil, where metal detection struggles.
- Product and Packaging Integrity Checks: X-ray systems can simultaneously perform multiple quality checks in a single pass, enhancing efficiency and accuracy. This includes:
- Missing Component Detection: Identifying missing tablets or capsules in a blister pack, or a missing leaflet/dosing spoon in a package.
- Fill Level Checks: Verifying the correct volume of liquids or the count of tablets in bottles/vials.
- Product Damage: Detecting broken or chipped tablets.
- Seal Integrity: Identifying foreign matter or product caught in the seals of packaging, which can compromise shelf life and sterility.
These comprehensive checks are vital for preventing costly and brand-damaging product recalls and for ensuring strict adherence to Good Manufacturing Practice (GMP) regulations.
Current & Upcoming Trends: The Age of Intelligent Inspection
The future of X-ray QA is being shaped by cutting-edge technological advancements, pushing the boundaries of what is possible on the production line.
Digital & Smart Systems
- High-Resolution Digital Imaging: The move from older film-based systems to digital imaging (Digital Radiography, Computed Tomography) offers superior clarity, faster processing, and easier data management.
- Integration with Industry 4.0: Modern X-ray systems are increasingly connected, providing real-time data collection and analysis. This facilitates process control, allowing manufacturers to quickly identify and correct upstream issues causing product defects.
The AI Revolution
The most significant trend is the integration of Artificial Intelligence (AI) and Machine Learning (ML):
- Enhanced Accuracy: AI algorithms are being trained to automatically recognize and classify even minute or complex defects, drastically reducing false rejects and increasing overall detection sensitivity.
- Spectral Unmixing: Advanced techniques, sometimes involving Dual-Energy X-ray (DEXA) technology, combined with AI, allow for better differentiation between materials of similar density, such as specific polymers or product irregularities, making contaminant identification more precise.
- Explainable AI (XAI): As AI takes on more critical roles, XAI tools are emerging to ensure the system’s decision-making process is auditable and transparent, a necessity in the highly regulated pharmaceutical environment.
Expert Opinion: “An Invaluable Asset”
Mike Pipe, Head of Global Sales for Mettler-Toledo Safeline X-ray, emphasizes that X-ray inspection is becoming an “invaluable asset” for pharmaceutical companies. “Manufacturers are waking up to how useful X-ray inspection can be, not just for catching contaminants, but for full product and packaging integrity checks that improve matters of cost, efficiency, and waste,” he states.
Another expert from the industry highlights the changing regulatory landscape: “As global pharmacopeia and regulatory bodies tighten their focus on physical hazards in drugs, the multi-functionality of X-ray provides a single, high-speed solution to address multiple compliance points simultaneously.” The digital record-keeping capabilities of modern systems also significantly ease the burden of regulatory audits and compliance validation.
Implications for Pharma Quality and Safety
The widespread adoption of advanced X-ray inspection has profound implications for the pharmaceutical sector:
- Heightened Patient Safety: By serving as the final line of defense on the production line, X-ray systems dramatically lower the risk of contaminated or structurally compromised medication reaching the consumer.
- Brand Protection and Cost Reduction: Fewer recalls due to foreign body contamination or incomplete packaging directly translates to protecting a manufacturer’s brand reputation and saving millions in recall-related expenses, legal costs, and inventory write-offs.
- Streamlined QA Processes: The ability to perform multiple, non-destructive checks at high line speeds eliminates the need for redundant, slower inspection steps, thus improving production throughput and operational efficiency.
In an industry where a single defect can have catastrophic consequences, X-ray inspection is no longer just an alternative; it is fast becoming the gold standard for proactive, intelligent quality assurance, ensuring that the integrity of life-saving medicines is protected from the inside out.