The travel of a pill from the fabricating line to a patient’s hand is a complex, worldwide journey full with chance. In an age of advanced forging, sedate redirection, and expanding e-commerce dangers, the keenness of the pharmaceutical supply chain has never been more crucial. The industry’s authoritative reply? Serialization and Track-and-Trace (T&T) frameworks, which are on a very basic level changing how medications are confirmed, followed, and protected.
This in-depth see investigates the vital part these innovations play in quiet security, the chronicled drive behind their appropriation, the current innovative scene, and the transformative suggestions for the worldwide pharmaceutical sector.
Background and Verifiable Setting: The Need of Transparency
The thrust for strong T&T frameworks is established in a decades-long fight against a deadly worldwide dark showcase. The World Wellbeing Organization (WHO) has evaluated that a critical rate of medications in moo- and middle-income nations are substandard or distorted (SF). These unlawful drugs can contain poisonous substances, inaccurate dynamic fixings, or none at all, driving to treatment disappointment, quiet hurt, and indeed passing. High-profile episodes, such as the 2007-2008 defilement of an debased blood more slender that caused various passings in the US, underscored the critical require for end-to-end supply chain visibility.

The First light of Advanced Traceability
Historically, item confirmation depended on clump numbers and anti-tampering seals, which were effectively circumvented by advanced hoodlums. The move toward advanced T&T started with early administrative efforts.

- Early Pioneers (2000s): Nations like Turkey and Italy were among the to begin with to build up comprehensive, national-scale T&T frameworks. Turkey’s Pharmaceutical Track & Follow Framework (ITS), rolled out broadly by 2012, got to be a worldwide show for its end-to-end traceability from the producer to the patient.
- Characterizing the Center: The cutting edge arrangement includes a two-part process:
- Serialization: The act of allotting a special identifier to the littlest saleable unit of a medicate (e.g., a bottle or carton). This interesting code, ordinarily a 2D Information Network standardized identification, incorporates the Worldwide Exchange Thing Number (GTIN), bunch number, close date, and a particular serial number.
- Track-and-Trace: The framework of checking and recording the development and value-based history of that serialized unit all through the supply chain. Following finds the product’s current and past areas, whereas Following recognizes all substances (producers, wholesalers, drug stores) that have taken care of it.
Regulatory Milestones
The need of worldwide standardization rapidly driven to required directions in major markets:
- The US Sedate Supply Chain Security Act (DSCSA) (2013): Diagrams a staged, national framework for electronic, interoperable T&T by 2023, requiring item serialization at the unit level.
- The EU Distorted Solutions Order (FMD) (2019): Requires a one of a kind identifier on medicine solutions and a framework for confirming item realness at the point of dispensing.
Current Patterns and Innovative Landscape
Modern T&T arrangements are leveraging progressions in computerized innovation to make strong and straightforward supply chains.
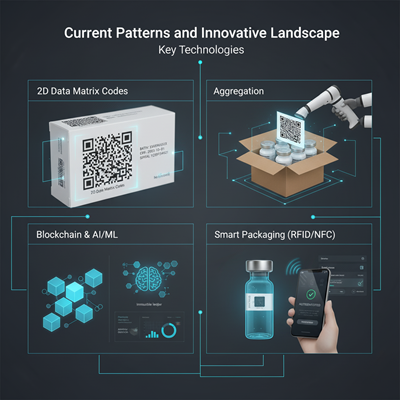
Key Technologies
- 2D Information Network Codes: The industry standard for serialization, giving a high-density, machine-readable arrange that stores the interesting item data.
- Conglomeration: A basic step where the serial numbers of littler units (e.g., bottles) are carefully connected to the serial number of the bigger bundling unit (e.g., a case or bed). This permits for proficient, bulk checking and confirmation, streamlining logistics.
- Blockchain: Developing as a transformative innovation, blockchain offers a decentralized, unchanging record for exchange information. Specialists see it as the another step for T&T, possibly upgrading information security, believe, and real-time confirmation over dissimilar partners without depending on a single, centralized authority.
- Shrewd Bundling (RFID/NFC): Radio Recurrence Recognizable proof (RFID) and Close Field Communication (NFC) labels are being progressively received, especially for high-value or strength drugs. These permit for contactless perusing of item data, advertising more prominent mechanization and real-time stock management.
- AI and Machine Learning (ML): These instruments are being coordinates to upgrade information analytics, empowering prescient observing to identify and hail irregularities or suspicious exercises in real-time some time recently a fake item can penetrate the genuine supply chain.
Expert Conclusions: A Digital-First Future
Supply chain security specialists emphasize that compliance is as it were the beginning point. The long-term esteem lies in turning T&T information into a vital commerce asset.
“The genuine payoff of serialization isn’t fair compliance; it’s the information open. Real-time traceability information, once coordinates with operational frameworks, drives major efficiencies—from focused on reviews to endlessly made strides stock administration and request determining,” says one industry consultant.
The rise of pharmaceutical e-commerce and the related danger of unlawful online medicate venders is a major concern, making strong verification at the point of dispensing—whether a physical drug store or an authorized online distributor—an supreme need. The agreement is that a digital-first, end-to-end perceivability demonstrate, built on standardized information, is non-negotiable for future security and resilience.
Suggestions for the Pharmaceutical Industry
The execution of T&T is causing a worldview move, making both critical challenges and significant openings over the whole pharmaceutical ecosystem.
Patient Security and Open Health
- Combating Forging: The essential and most pivotal suggestion is the creation of a capable obstruction against misrepresented drugs. By requiring each genuine item to have a one of a kind, irrefutable personality, it gets to be essentially incomprehensible to present a fake thing into the unquestionable supply chain.
- Focused on Reviews: T&T permits for surgical exactness amid item reviews. Instep of reviewing whole clumps or territorial shipments, companies can utilize the special serial numbers to recognize and disconnect as it were the particular units or items influenced, sparing time, lessening costs, and avoiding pointless sedate shortages.
Operational and Financial Impact
- Fetched and Complexity: The introductory taken a toll of executing serialization—upgrading fabricating lines, joining program, and overseeing enormous volumes of data—represents a significant speculation, especially for littler producers and Contract Fabricating Organizations (CMOs).
- Streamlined Coordinations: The unused frameworks drive a higher degree of supply chain straightforwardness and information sharing. Whereas challenging to actualize, this expanded perceivability leads to optimized stock control, diminished stockouts, minimized squander, and by and large more prominent operational efficiency.
- Brand Security: For producers, the capacity to ensure item realness at any point in the supply chain shields their brand notoriety and guarantees buyer trust.
Regulatory and Worldwide Harmonization
Serialization has constrained an phenomenal level of worldwide administrative arrangement, generally centered around the universal benchmarks set by GS1. As major economies command T&T, pharmaceutical companies working all inclusive must guarantee their frameworks are interoperable to handle assorted compliance necessities whereas keeping up a consistent stream of information for sends out and imports.
In conclusion, serialization and track-and-trace are no longer future concepts but the de facto gold standard for supply chain security. They serve as the computerized gatekeeper of pharmaceutical judgment, giving an unbroken chain of confirmation that ensures the wellbeing of both the quiet and the industry. Whereas the mechanical and budgetary speculation is noteworthy, the long-term return—in terms of quiet security, brand believe, and operational efficiency—is immeasurable.





