In the complex, high-stakes world of pharmaceutical enhancement, speed and around the world reach are crucial. A single authoritative reroute can delay life-saving medications and brought a company billions. Against this view, the Around the world Conference on Harmonization (ICH) E5 run the show, titled “Ethnic Factors in the Value of Exterior Clinical Data,” stands as a fundamental instrument for streamlining around the world sedate enrollment. By giving a framework to assess and minimize the influence of ethnic contrasts, ICH-E5 has essentially overhauled thing regard and animated the transport of unused drugs to patients around the world.
Background and Bona fide Setting: Wrapping up the “Steady Slack”
The starting of ICH-E5 in 1998 was a arrange response to the ponder known as the “cure slack,” particularly in districts like Japan. By and large, regulatory pros in particular regions—namely the U.S., Europe, and Japan (the interesting ICH regions)—often required add up to duplication of clinical trials interior their have specific populaces, undoubtedly if wide, high-quality data as of presently existed from other spaces. This mandatory emphasis through and through increased change timelines, extended costs, and delayed understanding get to to unused treatments.
The center challenge was tending to the potential affect of ethnic components on a drug’s ampleness, security, and perfect estimation. ICH-E5 characterized two crucial categories of these variables:
- Inherent Ethnic Components: Those related to genetic qualities and physiology, such as genetic polymorphisms that impact calm assimilation framework (Pharmacokinetics or PK), age, sexual introduction, and body mass.
- Outward Ethnic Factors: Those related with the environment and culture, checking thin down, smoking and alcohol utilize, co-medications, and the measures of helpful sharpen and disease conclusion in a given locale.
The guideline’s objective was fundamental in any case dynamic: to set up a coherently sound get ready for extrapolating clinical data from one region to another, along these lines minimizing the duplication of clinical considers around where ethnic affectability was moo.
The Instrument: Bridging and Extrapolation
ICH-E5 follows a urgent three-step handle for evaluating the value of exterior clinical data in a present day locale:
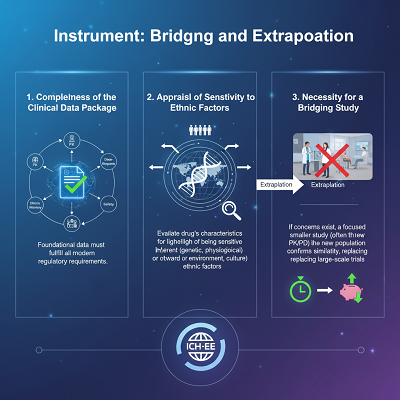
- Completeness of the Clinical Data Bundle: The foundational farther data must totally fulfill the common authoritative necessities for support in the cutting edge district, checking comprehensive data on PK, Pharmacodynamics (PD), dose-response, reasonability, and security.
- Appraisal of Affectability to Ethnic Factors: The drug’s innate characteristics are assessed to choose its likelihood of being sensitive to inalienable or outward ethnic factors. For event, a steady with a contract helpful window or one that is metabolized by an chemical with known genetic polymorphism is considered more delicate.
- Necessity for a Bridging Consider: If the clinical data is add up to but there is concern with regard to potential ethnic contrasts, a bridging think around may be required.
A bridging think almost is a centered, centered on examination performed in the unused region’s people to allow data that grants the extrapolation of the inaccessible clinical data. This consider is routinely small and is regularly a Pharmacokinetic (PK) or Pharmacodynamic (PD) consider to confirm that exposure-response associations are comparative, or possibly than a full, large-scale ampleness trial. By supplanting large-scale, duplicative Organize 3 trials with these more diminutive, centered considers almost, ICH-E5 drastically cuts down on the time and gotten of enrollment.
Current Designs and Ace Conclusions: The Around the world Change Worldview
Today, the measures of ICH-E5 are significantly embedded in the crucial orchestrating of multinational pharmaceutical companies, forming the foundation of around the world sedate headway (GDD).
Trend 1: Proactive, Comprehensive Advancement
The beginning center on post-hoc extrapolation has progressed into a incline of proactive, Asia-inclusive sedate headway. Or perhaps than making a calm in one major region (e.g., the U.S.) and at that point endeavoring to “bridge” to all others, companies by and by habitually arrange Multi-Regional Clinical Trials (MRCTs) from the begin. This is help maintained by the complementary ICH-E17 run the show on MRCTs.
- Master Understanding: Concurring to pharmacometric pros, tallying varying ethnic populaces in early-stage trials (Arrange 1 and 2) to assess PK and PD grants companies to gather ethnicity data proactively. This “totality of demonstrate” approach can habitually outline a drug’s relentlessness to ethnic components early on, conceivably invalidating the require for a apportioned bridging consider afterward.
Trend 2: The Portion of Quantitative Pharmacology
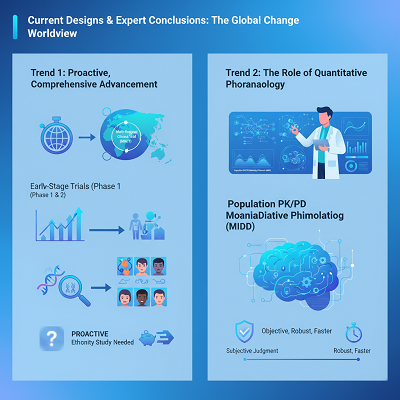
The examination of a drug’s affectability to ethnic components depends heightening on Quantitative Clinical Pharmacology. Advanced devices like people PK/PD modeling and reenactment (MIDD – Model-Informed Steady Change) are utilized to expect how contrasts in factors like innate assimilation framework might impact sedate introduction and response in a unused masses. This coherent meticulousness replaces subjective judgment, growing the certainty of regulatory bodies in enduring exterior data.
- Influence: The move toward quantitative data-driven decision-making has made the ICH-E5 handle more objective, overwhelming, and in the long run speedier.
Implications: Progressing Thing Regard and Determined Get to
The successful application of ICH-E5 has critical proposals over the pharmaceutical scene:
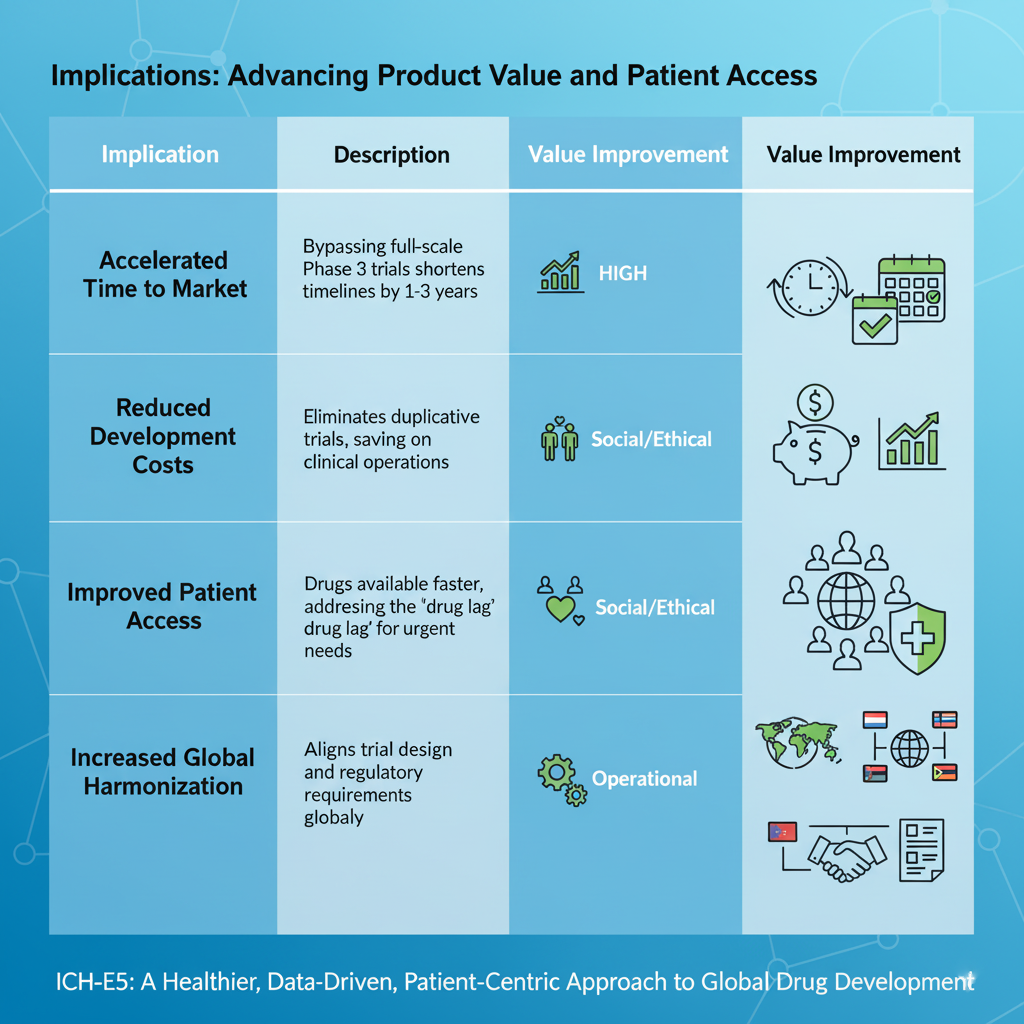
| Implication | Portrayal | Esteem Improvement |
| Accelerated Time to Advertise | By keeping up a vital separate from the require to go over full-scale Organize 3 clinical trials, advancement timelines for a present day region can be truncated by 1 to 3 a long time or more. | Tall: Maximizes the drug’s self-evident life and exhibit eliteness period, basically growing lifetime income. |
| Reduced Enhancement Costs | Dispensing with duplicative sweeping trials comes around in significant save reserves on clinical operations, specialist costs, and area checking. | Tall: Makes strides the return on wander (ROI) for steady enhancement, freeing up capital for unused R&D. |
| Improved Understanding Get to | Drugs finished up available in advanced regions much faster, tending to the issue of the “steady slack” and benefitting patients who have squeezing, ignored remedial needs. | Social/Ethical: Fulfills the industry’s ethical command and builds open believe. |
| Increased Around the world Harmonization | The benchmarks drive more conspicuous course of action in trial arrange and authoritative wants between arranged around the world masters, streamlining the way for a single, harmonized convenience (Common Specialized Record or CTD). | Operational: Increases viability for authoritative endeavors groups. |
ICH-E5 has progressed from a run the show arranged to enlighten a regional issue into a center imperative column for around the world sedate headway. Its inheritance is a more beneficial, tentatively grounded, and patient-centric approach to bringing life-saving drugs to the world faster.





