Olympus Organization has begun an basic, around the world ejection of specific packages of its ViziShot 2 FLEX (19G) EBUS-TBNA needles taking after diverse reports of contraption components isolating in the midst of fundamental biopsy methodologies. This around the world movement, doled out a Course I recall—the most veritable classification by the U.S. Food and Calm Organization (FDA)—was affected by security concerns, tallying distinctive point by point wounds and one calm passing associated to the flawed devices.
The event is the most later in a course of action of high-profile security issues for the restorative contraption mammoth, underscoring decided concerns roughly quality control in the exceedingly specialized field of insignificantly prominent surgical equipment.
Background and The Defect
The ViziShot 2 FLEX is an endoscopic objective needle utilized with ultrasound endoscopes for Endobronchial Ultrasound-Guided Transbronchial Needle Crave (EBUS-TBNA). This insignificantly meddling strategy is vital for diagnosing lung diseases, tallying organizing lung cancer, by allowing specialists to collect tissue tests from lymph centers and wounds in the tracheobronchial tree.
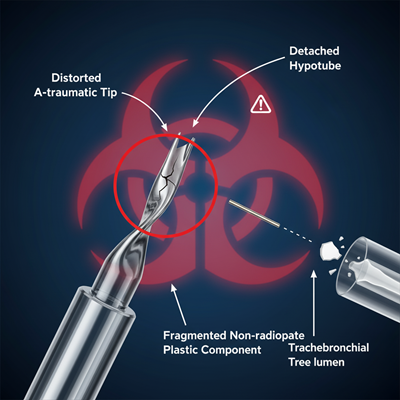
The Speedy Danger
The root of the issue lies with certain contraptions created prior to May 12, 2025, which may have a conceivably turned a-traumatic tip. This distortion basically increases the chance of component dispatch in the midst of use.
- Detached Components: The flaw can cause the hypotube (a protective metal tube) or pulled back plastic components to dispatch from the device.
- Patient Risk: These parts can enter a patient’s flying course (tracheobronchial tree). The plastic components are famously not radiopaque, meaning they are imperceptible on standard X-ray imaging, making provoke disclosure and recuperation enormously difficult.
- Adverse Comes about: The utilize of affected thing may cause honest to goodness harmed, checking mucosal harm, passing on, defilement, and passing. Clearing the held parts routinely requires additional prominent procedures, such as bronchoscopic extraction or surgical removal.
Olympus asserted getting unfavorable event reports counting tireless wounds and one passing related with the distortion, driving to the speedy clearing movement for all impacted portion numbers scattered between Honorable 2022 and April 2025.
Historical Setting: A Plan of Security Concerns
Olympus has a complex and regularly scrutinized history with regard to the security and quality of its therapeutic contraptions. Though it remains a overpowering drive in the around the world endoscope publicize, the company has stood up to basic authoritative and authentic challenges over the past decade related to contraption reprocessing and design.
Duodenoscope Crisis
The most recognizable true security issue included Olympus’s TJF-Q180V duodenoscopes. In the mid-2010s, these contraptions were associated to life-threatening “superbug” flare-ups in clinics around the world. The complex arrange of the scopes, particularly the versatile lift component at the tip, made them troublesome to clean and sterilize palatably, allowing infinitesimal living beings to be transmitted between patients.
The coming about regulatory examination and legal action obliged Olympus to pay gigantic fines and execute clearing changes to its quality control and arrange shapes. This history sets up a point of reference of security issues, habitually turning around manufacturing and arrange flaws in complex, irrelevantly meddling symptomatic rebellious. The current ViziShot 2 FLEX survey is a extraordinary, be that as it may specific, failure—shifting the center from cleaning traditions to the perception of the contraption components themselves.
Current Designs and Olympus’s Response
In response to the ViziShot 2 FLEX component division issue, Olympus has taken a number of medicinal actions:
- Global Clearing: An basic therapeutic contraption ejection letter was sent to all impacted clients, course them to instantly separate and return all contraptions with the affected portion numbers.
- Manufacturing Upgrade: Fundamentally, Olympus has made strides its quality control handle at the point of manufacture. The company supplanted its past manual, visual survey technique for distinguishing contraption hurt in the midst of gathering with a cutting edge robotized evaluation strategy. This move recognizes that the defect likely begun in the midst of the manufacturing process.
- Reinforced Takes note: Olympus is besides invigorating existing takes note in the device’s Edifying for Utilize (IFU), especially course clients not to influentially pushed the needle if resistance is experienced, as this appear contribute to contraption hurt and component ejection.
The FDA’s classification of this as a Lesson I audit signals the agency’s affirmation of the uncommon chance to open health.
Master Conclusions and Implications
The survey has basic proposals for patients, specialists, and the helpful contraption industry.
Patient Security and Remedial Practice
Experts in pneumonic and gastroenterological strategies extend the provoke require for carefulness. The reality that restricted plastic components are not radiopaque is a major clinical concern.
“The non-radiopaque nature of the parts is the quiet threat here,” clarifies a driving pulmonologist specializing in EBUS-TBNA (who inquired namelessness due to mending center approach). “In case a understanding presents with post-procedural side impacts like decided hack, fever, or breathing inconvenience, specialists must have a tall record of question for held inaccessible bodies, without a doubt if early on X-rays are clear. It powers us to consider more advanced, non-standard imaging or go over endoscopy.”
Clinics must directly exactingly track and organize of affected packages, and providers must be reminded that for certain high-risk strategies, like EBUS-TBNA, any seen resistance in the midst of the contraption incorporation should to be an speedy reddish accost to end and supplant the device.
Industry Examination and Corporate Accountability
This event re-ignites the conversation around over therapeutic contraption quality and post-market perception. The FDA’s Course I task is a able open hail that Olympus’s past quality control measure—a visual inspection—was deficiently for a contraption with life-or-death consequences.
The recommendations for Olympus are budgetary and reputational.
- Financial Influence: The taken a toll of the around the world audit, credit for returned contraptions, and potential true blue liabilities from the nitty gritty passing and wounds will be substantial.
- Reputational Hurt: For a company that has unreservedly communicated its commitment to understanding security taking after the duodenoscope crisis, a major manufacturing-related Lesson I survey so some time recently long after is a extraordinary blow to its legitimacy. It raises vital questions around the practicality of its “Lift” quality alter program, impelled to fortify its understanding security focus.
The required utilization of an computerized appraisal system by Olympus sets a likely unused industry standard for the create of complex, sensitive biopsy needles, as a manual visual check is directly illustrated to be an inadmissible danger. The industry, and particularly Olympus, will be underneath unequivocally regulatory weight to outline that their quality organization systems are solid adequate to expect such deplorable disillusionments in the future.





