The European pharmaceutical scene is experiencing a significant change, driven by uncommon logical propels, mounting financial weights, and yearning administrative upgrade. For pharmaceutical companies, key route is no longer a matter of incremental alter but a survival basic. This in-depth examination dives into the foundational setting, basic current patterns, and the key suggestions of the moving sands in the world’s second-largest pharmaceutical market.
Background and Authentic Context

The establishment of the advanced European pharmaceutical advertise is established in a post-Thalidomide world, where the essential driver got to be quiet security and viability. Key points of reference include:
• Early Orders (1960s-1970s): Taking after the Thalidomide catastrophe, the European Financial Community (EEC) started presenting system enactment, such as Order 65/65, to harmonize national directions and set up benchmarks for showcasing authorization.
• The Single Showcase and the EMA (1990s): The creation of the European Single Advertise required a more centralized approach. The European Medications Office (EMA) was set up in 1995, advertising centralized and decentralized methods for medicate endorsement, essentially streamlining get to to the EU showcase. This was a basic step in making a bound together showcase, in spite of the fact that cost and repayment choices remained—and still to a great extent remain—at the national level.
• Focus on Neglected Needs (2000s onwards): Consequent enactment centered on incentivizing R&D in specialized ranges, outstandingly with the Vagrant Sedate Control (2000) for uncommon illnesses and committed directions for solutions for children and Progressed Treatment Therapeutic Items (ATMPs), such as quality and cell therapies.
This history has developed a framework characterized by tall security guidelines and a collective health-system center, however too by fracture in estimating and showcase get to, which has progressively slacked behind the pace of development seen in the US market.
Current Patterns and Characterizing Challenges

The European pharma showcase nowadays is characterized by a division: taking off logical opportunity against seriously commercial and administrative pressure.
Key Patterns Forming the Landscape
1. Scientific Breakthroughs and Specialization:
o Advanced Treatments (ATMPs): Quality and cell treatments are at the cutting edge, requiring unused administrative direction (like the EMA’s PRIME plot for quickened back) and novel repayment models due to their tall taken a toll and potential for cure.
o Precision and Personalized Pharmaceutical: Fitting medications based on person hereditary qualities, especially in Oncology and Immunology, is driving the most noteworthy development. Oncology remains the best treatment region all inclusive, and request for anti-obesity medicines is anticipated to skyrocket, moving into the best five treatment zones by 2030.
o Digital Wellbeing and AI: The integration of Fake Insights (AI) in R&D, diagnostics, and persistent administration is quickening medicate revelation. Companies are leveraging AI to optimize clinical trials and light up covered up commercial insights.
2. Regulatory Redesign and Get to Focus:
o The EU Pharmaceutical Procedure and Bundle: The current, gigantic update of the EU’s common pharmaceutical enactment is the most critical administrative alter in decades. Its center objectives are to guarantee evenhanded and opportune get to to reasonable solutions over all Part States, improve the security of supply (tending to deficiencies), and advance innovation.
o Pricing and Repayment Weights: Governments and payers proceed to look for cost-effective arrangements. The modern enactment proposes to decrease the standard period of administrative information assurance for modern medications, with expansions allowed as it were if companies dispatch the item in all EU Part States inside two a long time or meet tall neglected therapeutic needs. This is a coordinate degree to quicken generic/biosimilar section and thrust for pan-EU access.

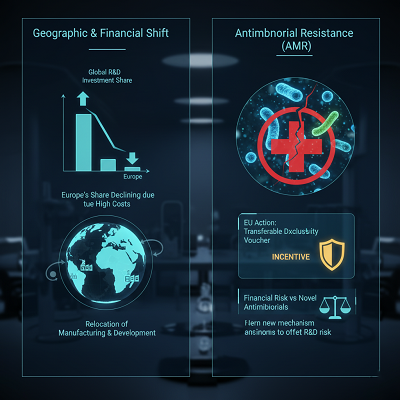
3. Geographic and Financial Shift:
o Loss of Worldwide R&D Share: Europe’s share of worldwide pharmaceutical deals and R&D speculation has been slowly moving to North America and fast-growing rising economies like China. Tall generation costs, vitality costs, and expanding administrative burdens are driving a few major companies to consider migrating development and generation capacities abroad.
o Antimicrobial Resistance (AMR): This is a basic open wellbeing emergency. The EU is taking definitive activity by proposing a transferable information eliteness voucher to incentivize the advancement of novel antimicrobials, offsetting the money related chance related with prudent-use guidelines.
Expert Suppositions and Key Implications
Experts to a great extent concur that the industry is at a major emphasis point where existing commerce models are being challenged by administrative arrangement planned to prioritize open wellbeing equity.
The Advancement vs. Get to Debate
The proposed lessening in administrative assurance is the most disagreeable point.
“The shortening of administrative security period would clearly quicken the advancement and endorsement of generics and biosimilars, hence accomplishing broader and more reasonable get to to novel drugs,” notes one industry investigator. “In any case, the result… may be that companies will see less commercial esteem in creating certain imaginative drugs and will be constrained to forsake the more unsafe projects.”
This makes a high-stakes trade-off: The approach points to fathom a social issue (get to and reasonableness) but dangers compounding a commercial one (reduced R&D incentive).
Strategic Route for Pharma Companies
For trend-setters, the way forward requires a move from a product-centric to an ecosystem-centric strategy:
| Strategic Pillar | Actionable Imperatives |
| R&D Focus | Prioritize High-Unmet Need: Invest in areas (like specific cancers, rare diseases, or novel antimicrobials) that will qualify for extended regulatory protection under the new EU rules. Leverage AI and Real-World Evidence (RWE) to accelerate clinical development and demonstrate patient value beyond traditional endpoints. |
| Market Access | Adopt a Pan-EU Launch Strategy: To secure maximum exclusivity, companies must plan for simultaneous or near-simultaneous launch across all Member States. This requires massive logistical and strategic coordination to navigate disparate national pricing and reimbursement (P&R) negotiations. |
| Supply Chain | Enhance Resilience and Localization: The focus on supply security necessitates diversifying supply chains and potentially re-shoring or near-shoring the manufacturing of critical medicines within the EU. Digital twin technology and advanced analytics will be crucial for optimizing supply logistics. |
| Compliance and Transparency | Embrace Green and Digital Requirements: Companies must align operations with the environmental sustainability standards of the European Green Deal. Furthermore, the increasing demand for public disclosure of public R&D subsidies requires greater financial transparency in drug development. |
The move towards Harmonized Wellbeing Innovation Appraisal (HTA) at the EU level, set to come into full impact, will too streamline the prove required to illustrate clinical esteem. Companies that can rapidly and heartily create the information required for this joint clinical appraisal will pick up a basic advantage in national P&R processes.
Conclusion:
The advancing European pharmaceutical scene is one of calculated chance and colossal opportunity. The EU’s administrative change is an unequivocal endeavor to use the single market’s control to implement open wellbeing goals—namely, get to and affordability.
For pharmaceutical companies, the challenge is clear: Advancement alone is no longer sufficient; it must be coupled with an forceful, pan-European get to methodology. Firms that effectively coordinated logical breakthroughs (ATMPs, AI), operational versatility (supply chain security), and versatile commercial models that adjust with the EU’s unused access-focused arrangement will be the ones to effectively explore this complex territory and secure their future in a showcase on a very basic level committed to widespread, reasonable healthcare.





