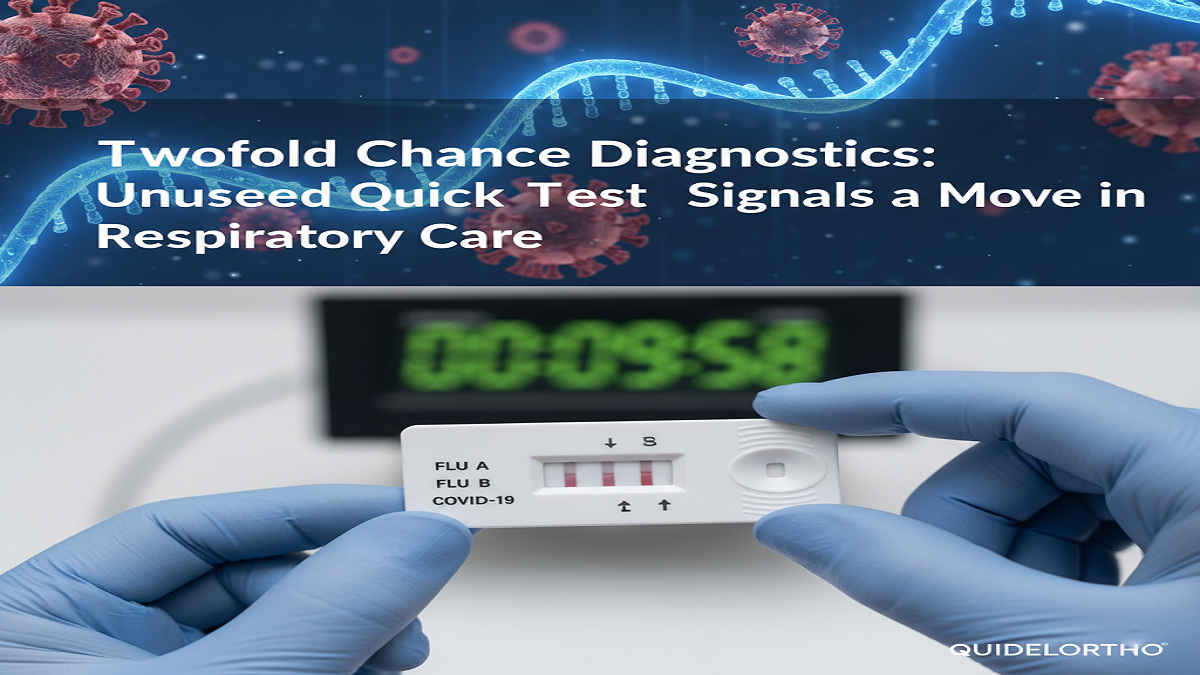
In a vital movement for respiratory ailment organization, QuidelOrtho Venture has announced the openness of its QUICKVUE Flu + SARS Test, a professional-use fast definite that can at the same time recognize and isolated between Flu A, Flu B, and SARS-CoV-2 (the disease that causes COVID-19) from a single understanding test. This CLIA-waived, 510(k)-cleared immunoassay gives comes approximately in as little as 10 minutes, a speed that might altogether streamline clinical decision-making in the midst of best respiratory seasons.
The alacrity of this “triple area” test—which does not require a investigate office instrument and offers a ostensibly inspected result—is a crucial expansion of QuidelOrtho’s incredible QUICKVUE portfolio and underscores a major float in diagnostics: the move toward beneficial, multiplexed, point-of-care (POC) testing.
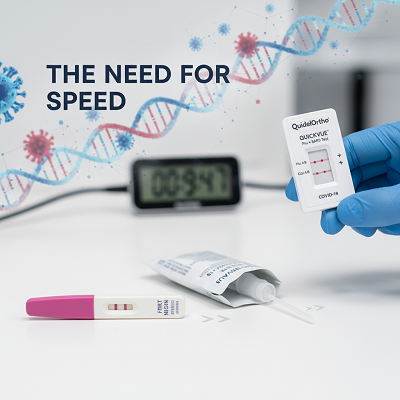
Background and Unquestionable Setting: The Require for Speed
The advancement of speedy expressive tests (RDTs) for powerful contaminations is not unused, taking after its roots back to essential, color-based immunoassays like the cutting edge household pregnancy test. In any case, the criticalness and complexity of respiratory testing increased radically with the COVID-19 pandemic.
The Progression of Flu and SARS Testing
- Early Flu RDTs (RIDTs): Fast Flu Symptomatic Tests (RIDTs) have been utilized broadly since the 1990s, promoting quick comes almost (frequently interior 15 minutes) for flu A and B. Their crucial impediment genuinely was a tolerably lower affectability compared to the “gold standard” of viral culture or, a short time later, nuclear testing (RT-PCR). In 2017, the FDA renamed RIDTs, requiring higher slightest measures for performance.
- The SARS-CoV-2 Catalyst: The onset of the COVID-19 broad saw an unprecedented around the world pushed to make quick, decentralized testing. Starting endeavors centered on single-target tests (on a very basic level SARS-CoV-2), regularly utilizing Emergency Utilize Authorization (EUA) pathways to quickly reach the market.
- The Symdemic Challenge: As COVID-19 and customary flu begun to co-circulate, healthcare providers gone up against a essential symptomatic pickle: the side impacts of the two diseases are approximately vague, in any case their drugs and open prosperity proposals move basically. This “symdemic” reality made continuous or confined testing—requiring two swabs or two unmistakable tests—inefficient, slanted to botch, and resource-intensive. This made an speedy, strong ask for multiplex measures that might assert or run the appear out both pathogens simultaneously.

The QuidelOrtho QUICKVUE Flu + SARS Test clearly addresses this require by giving a visually-read, significantly accessible POC course of action that bypasses the time and complexity of customary lab-based nuclear testing.
Current Designs: Multiplexing and Decentralization
The unused QUICKVUE test alters immaculately with two overpowering designs shaping the cutting edge symptomatic landscape:
- Multiplexing is the Cutting edge Standard: The time of single-pathogen testing for common respiratory sicknesses is rapidly obscuring. Healthcare systems are grasping tests that recognize a board of respiratory contaminations, checking Flu A/B, SARS-CoV-2, and continuously, Respiratory Syncytial Contamination (RSV). This viability grants for faster and more correct syndromic assurance, saving resources and ensuring supply chains.
- Point-of-Care (POC) Decentralization: The incline towards CLIA-waived tests—tests direct adequate to be utilized in non-laboratory settings—continues its vitality. By being CLIA-waived, the QUICKVUE test can be sent in a colossal orchestrate of settings, checking specialist office investigate offices (POLs), squeezing care centers, emergency workplaces, and retail medicate stores. This decentralization moves conclusion closer to the calm, engaging quick “test-and-treat” models.
Expert Conclusions and Clinical Value
Clinicians and open prosperity pros overwhelmingly see combined speedy tests as essential for compelling respiratory sickness organization, especially in the midst of the colder months when both flu and COVID-19 cases surge.

“Physician office labs and squeezing care centers are on the front lines of respiratory ailment conclusion,” communicated a QuidelOrtho specialist upon the test’s celerity. “This advancement reflects QuidelOrtho’s commitment to supporting healthcare specialists with cost-effective courses of action that streamline clinical choices and offer help supervise standard surges in respiratory infections.”
The provoke clinical benefits are clear:
- Timely Treatment Choices: For both flu and COVID-19, early assurance is essential. Antiviral drugs like Tamiflu (for flu) are most effective when overseen interior 48 hours of sign onset. A 10-minute result licenses for fast medication, conceivably directing genuine affliction and hospitalization.
- Infection Control: Speedy partition makes a distinction prosperity systems make speedy defilement control choices, such as which isolation traditions to apply to the understanding and whether to begin contact taking after for COVID-19.
- Reduced Resource Strain: Utilizing one swab and one test pack instep of two moderates important resources like reagents and PPE, and lessens the definitive burden on front-line staff.
Implications for Healthcare and Open Health
The FDA clearance of the QuidelOrtho QUICKVUE Flu + SARS Test carries critical proposals over the healthcare ecosystem:
- Imperative Publicize Position
The present day test reinforces QuidelOrtho’s competitive advantage in the POC respiratory testing exhibit. By promoting a visually-read, instrument-free elective, it gives a cost-effective elective to the company’s existing instrument-based multiplex test, the SOFIA 2 Flu + SARS Antigen FIA. This twofold promoting caters to workplaces with changing budgets and specialized capabilities.
- Moving forward Community Surveillance
Wider, speedier choice of such tests at the community level—particularly in pharmacies—will move forward viral observation. Advantageous, isolated data on the co-circulation of flu and COVID-19 is invaluable for open prosperity specialists, allowing them to prevalent allocate antibodies, antivirals, and mending center resources.
- Shaping Understanding Behavior
The test’s consolation energizes symptomatic individuals to see for testing sooner. A clear, quick result—whether it’s the flu, COVID-19, or neither—empowers patients to manage their affliction, take appropriate partition measures, and get to crucial treatment quickly, in this way bringing down the risk of forward transmission and extraordinary outcomes.
In an environment where respiratory afflictions remain a consistent around the world burden, the move to quick, triple-detection testing is an significant instrument in the open prosperity weapons store. The QUICKVUE Flu + SARS Test is more than reasonable a unused thing; it is a significant appearance of lessons learned in the midst of the broad, promising a more successful and responsive future for respiratory diagnostics.