Human Challenge Trials (HCTs)—medical ponders where solid, educated volunteers are intentioned uncovered to a pathogen—represent one of present day medicine’s most powerful, however disputable, devices. In the race to create immunizations and medicines for irresistible infections, especially amid fast-moving pandemics, these considers offer a special alternate route, building up an early risk-benefit profile for novel countermeasures.
Background and Component: A Controlled Infection
At their center, HCTs, too known as Controlled Human Contamination Models (CHIMs), are planned to compress the timeline of conventional clinical advancement. In a standard Stage 3 viability trial, analysts must hold up for a adequate number of members to be normally uncovered to the infection to decide if a antibody works. This can take months or indeed years.
HCTs bypass this instability. Sound, altogether screened volunteers—typically youthful grown-ups with no basic wellbeing conditions—are vaccinated with a carefully arranged, for the most part well-characterized irresistible specialist (the “challenge stock”) after getting a candidate immunization or a fake treatment. The whole handle is conducted in a profoundly controlled, frequently isolated, inpatient setting with 24/7 therapeutic supervision.

Key Advantages:
- Speed: They can quickly set up proof-of-concept and give early adequacy information, quickening the choice to development or drop a immunization candidate.
- Clarity: Analysts pick up exact information on the timing of contamination, viral stack energy, and the safe reaction from the most punctual conceivable minute, which is important for understanding illness pathogenesis and recognizing connects of assurance (resistant markers that anticipate security from disease).
- Efficiency: They require distant less members than conventional field viability trials.
Historical Setting: From Jenner to Cutting edge Scrutiny
The hone of deliberateness disease for therapeutic advantage is not modern; it follows its roots back to the exceptionally establishment of vaccinology.
Early, Unscrupulous Beginnings
The verifiable record incorporates both basic turning points and terrible moral failures:
- Smallpox and Cowpox (1796): English doctor Edward Jenner’s foundational work on immunization included intentioned vaccinating a youthful boy with cowpox and at that point uncovering him to smallpox, setting up immunity.

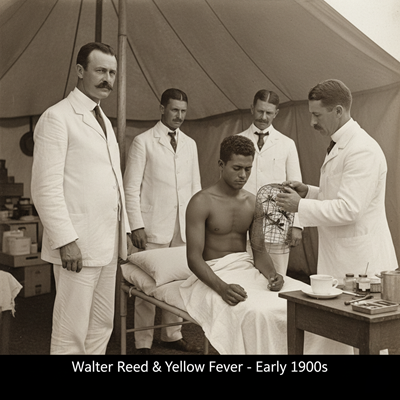
- Yellow Fever (Early 1900s): Trials conducted by the U.S. Armed force in Cuba, driven by Walter Reed, included permitting tainted mosquitoes to nibble volunteers, authoritatively demonstrating that mosquitoes transmit yellow fever. Whereas deductively effective, these early trials regularly included lacking assent and abused powerless populaces, reflecting a darker time of restorative research.
- Mid-20th Century Mishandle: Cases like the Tuskegee Syphilis Consider and purposefulness hepatitis disease considers on institutionalized children serve as stark updates of verifiable infringement, for all time forming the cutting edge moral and administrative landscape.
The Cutting edge, Controlled Era
Today’s HCTs work beneath exacting worldwide and national moral rules, established in codes like the Nuremberg Code and the Announcement of Helsinki. Over the past a few decades, HCTs have been instrumental in creating and quickening antibodies for maladies like jungle fever, cholera, typhoid fever, and flu, setting up a modern track record of security when connected to illnesses with moo chance of genuine sickness and a solid treatment or protect therapy.
Current Patterns: The Widespread Accelerator
The COVID-19 widespread essentially heightens the talk about and utilize of HCTs.
The SARS-CoV-2 Model
Despite introductory moral concerns over the need of a demonstrated protect treatment early in the widespread, the worldwide wellbeing crisis incited the first-ever human challenge trial for SARS-CoV-2. The UK’s COVID-19 Human Challenge Program, for occurrence, securely contaminated youthful, solid volunteers with an early-stage variation of the virus.

These considers were vital not for last antibody authorizing, but for:
- Determining the least irresistible dose.
- Gaining uncommon, point by point knowledge into the full course of disease, from introductory introduction to viral shedding, which educated open wellbeing approach (such as the unwavering quality of Sidelong Stream Tests).
- Providing a standardized, solid stage to quickly test next-generation antibody and helpful candidates against modern variants.
Expanding Portfolio
Beyond COVID-19, HCTs are a major portion of worldwide endeavors to handle other high-impact pathogens:
- Malaria: HCTs, regularly including the nibble of contaminated mosquitoes, stay a key step in screening unused antibody candidates, counting the advancement of the RTS,S vaccine.
- Respiratory Syncytial Infection (RSV): Challenge models are utilized to get it the safe reaction to this common, however unsafe, pediatric and geriatric virus.
- Tuberculosis (TB): Analysts are effectively creating a secure TB challenge demonstrate to quicken the improvement of frantically required modern TB vaccines.

Expert Conclusions and Moral Implications
The characterizing highlight of cutting edge HCTs is the cautious adjust between social esteem and person risk.
The Risk-Benefit Calculus
Experts overwhelmingly concur that HCTs are morally reasonable as it were when certain conditions are met:
| Criterion | Rationale |
| High Social Value | The information picked up must be experimentally fundamental and give a self evident open wellbeing advantage that cannot be gotten as rapidly or viably by other implies (e.g., quickening immunization arrangement in a pandemic). |
| Low Hazard to Participants | The pathogen must be well-understood, ordinarily causing gentle or direct illness in the target populace (solid youthful grown-ups). The consider must utilize an constricted (debilitated) strain if possible. |
| Availability of Protect Therapy | An successful treatment must be accessible to instantly remedy the contamination or anticipate extreme malady progression. |
| Robust Educated Consent | Volunteers must be completely advised of all potential dangers, counting uncommon or obscure extreme results, and must assent deliberately, free from coercion. |
Expert Knowledge: Bioethicists frequently emphasize that the moral defense shifts from ensuring the quiet to maximizing societal advantage. As Daniel Hausman, a investigate teacher at Rutgers Center for Population-Level Bioethics, famous amid the COVID-19 wrangles about, the potential to spare thousands of lives can morally legitimize the inconvenience of little dangers on a few well-informed volunteers.
Ongoing Moral Challenges
Despite shields, challenges persist:
- The “Obscure Questions“: For novel infections like SARS-CoV-2, the full range of hazard (e.g., long-term sequelae like “Long COVID”) is at first obscure, complicating the educated assent process.
- Generalizability: Since members are chosen to be low-risk, their safe reaction may not precisely reflect that of high-risk populaces (the elderly, those with comorbidities), restricting the study’s generalizability for licensing.
- Undermining Believe: A serious unfavorable occasion in an HCT may seriously disintegrate open believe in clinical inquire about and immunization advancement globally.
Implications for the Future
The utility of HCTs is anticipated to develop as portion of worldwide widespread readiness techniques. Administrative bodies like the World Wellbeing Organization (WHO) recognize their esteem as a key apparatus for quickly down-selecting the most promising immunization candidates in the confront of an rising threat.
The future will likely see:
- Establishment of Challenge Stock Libraries: Pre-approving and keeping up a bank of secure, characterized challenge strains for high-priority pathogens will permit for an indeed speedier reaction to modern outbreaks.
- Harmonized Worldwide Directions: Expanded collaboration among worldwide controllers to standardize the moral and fabricating prerequisites for HCTs, especially for trials conducted in moo- and middle-income nations where illness burden is highest.
In the interminable arms race against irresistible infections, Human Challenge Trials—conducted with fastidious moral rigor—remain a crucial, calculated chance basic for quickening the improvement of life-saving immunizations and treatments. They are a significant show of human charitableness, depending on volunteers who step forward to intentioned confront a illness for the wellbeing of the more extensive world.