In the burgeoning field of quality treatment, a progressive course of medication that treats illness by altering a patient’s hereditary fabric, the travel from a groundbreaking logical disclosure to a usable helpful item is full with challenges. The most basic and complex portion of this travel is the move from “quality to GMP” (Great Fabricating Hone), which includes scaling up laboratory-based investigate forms into a reliable, secure, and compliant fabricating operation. This crossroads is where the guarantee of quality treatment meets the down to earth substances of industrial-scale production.

A See Back: From Lab Seat to Bedside
The history of quality treatment has been stamped by both mind blowing breakthroughs and critical mishaps. The conceptual foundation was laid in the 1970s with the approach of recombinant DNA innovation. By 1990, the to begin with formally affirmed quality treatment trial took put, treating a youthful young lady with adenosine deaminase insufficiency (ADA-SCID), a serious resistant disorder.
However, the field experienced a “dim age” in the early 2000s after a understanding passed on from an provocative resistant reaction to a viral vector, and a few children in a isolated trial created a leukemia-like condition. These occasions driven to a end in numerous trials and a period of seriously investigation. It was from this cauldron that the advanced time of quality treatment fabricating developed, driven by moved forward viral vector plan and a recharged center on security and vigorous fabricating practices.
Today, quality treatment is encountering a renaissance. The U.S. Nourishment and Sedate Organization (FDA) has endorsed numerous quality treatments, counting those for conditions like spinal solid decay (SMA) and certain acquired retinal infections. This victory, in any case, has brought the “quality to GMP” challenge to the forefront.

The Center Challenges: A Group of three of Complexity
The deterrents in quality treatment fabricating are on a very basic level diverse from those of conventional small-molecule drugs or biologics. They can be categorized into three primary ranges: specialized complexity, calculated obstacles, and administrative ambiguity.
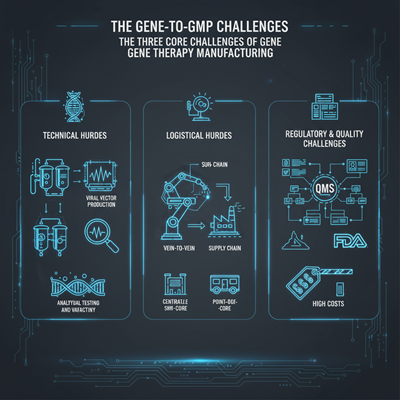
Technical Hurdles
- Viral Vector Generation: The endless larger part of quality treatments depend on viral vectors, such as adeno-associated infections (AAV) and lentiviruses (LV), to convey the helpful quality. Fabricating these vectors is a organic handle, not a chemical one, making it inalienably variable. Getting reliable, high-titer yields of high-quality vectors with a moo proportion of “purge” capsids (viral shells without the hereditary payload) is a major specialized challenge.
- Analytical Testing: Guaranteeing the virtue, power, and personality of these living items is colossally troublesome. Not at all like a chemical compound that can be effortlessly analyzed, a quality therapy’s power is frequently tied to its natural work, requiring complex, time-consuming tests that can take months to create and validate.
- Lack of Standardization: There is no one-size-fits-all fabricating stage. Each modern quality treatment frequently requires a bespoke approach, from cell lines to decontamination strategies, driving to a divided and siloed industry.
Logistical Hurdles
- Supply Chain: The supply chain for quality treatment is delicate and complex. Crude materials, counting plasmids and cell lines, must meet rigid quality measures. Besides, autologous treatments, which utilize a patient’s claim cells, require a “vein-to-vein” calculated chain with near-perfect coordination to guarantee the patient’s cells are collected, adjusted, and returned securely and on time.
- Decentralization vs. Centralization: The industry is hooking with whether to construct expansive, centralized fabricating offices or littler, more spry “point-of-care” locales closer to the persistent. Each show has its possess trade-offs in terms of taken a toll, adaptability, and calculated risk.
Regulatory and Quality Challenges
- Evolving Directions: The FDA and other administrative bodies are still creating their systems for this modern course of medication. This makes a moving target for producers who must ceaselessly adjust their forms and documentation to meet advancing Great Fabricating Hones (GMP). Vigorous documentation and a solid Quality Administration Framework (QMS) are paramount.
- High Costs: The complexity and bespoke nature of the fabricating prepare, coupled with the costly crude materials and foundation, contribute to the sky-high taken a toll of these treatments, regularly coming to millions of dollars per measurements. This postures a critical boundary to persistent get to and commercial viability.
Expert Conclusions and Current Trends
Industry pioneers and specialists are effectively working to address these challenges. A major slant is the move toward mechanization and closed frameworks. By decreasing manual mediation, computerization minimizes the chance of defilement and makes strides item consistency and reproducibility.
Many companies are too grasping vital organizations with contract improvement and fabricating organizations (CDMOs). These collaborations permit littler biotech firms to use the ability and framework of experienced producers, quickening their travel from clinical advancement to commercialization.
Experts moreover emphasize the require for a “quality-by-design” approach, where fabricating forms and controls are considered from the most punctual stages of improvement, or maybe than as an idea in retrospect. This proactive approach can anticipate exorbitant bunch disappointments and delays down the line.
The Suggestions: A Move in Healthcare Paradigms
Successfully exploring the “quality to GMP” challenges has significant suggestions. For patients, it implies a more dependable supply of life-saving treatments and, eventually, more available treatment choices. For the healthcare framework, it guarantees a move from indication administration to a healing demonstrate for hereditary diseases.
However, the suggestions moreover amplify to the commerce demonstrate of biopharma. The tall taken a toll of fabricating and the little understanding populaces for numerous uncommon maladies require unused approaches to estimating, repayment, and commercialization. The tall taken a toll, combined with the one-time nature of numerous quality treatments, is constraining payers and policymakers to re-evaluate conventional installment models.
In conclusion, the travel from a restorative quality to a commercially reasonable item is a complex and capital-intensive endeavor. Whereas noteworthy challenges stay, a concerted exertion over the industry, upheld by advancement in fabricating innovation and advancing administrative direction, is clearing the way for a future where quality treatment is not fair a logical wonder, but a broadly accessible reality.