The clinical trial scene is characterized by instability, eccentrics, and tall fetched. From fluctuating understanding enrollment and unanticipated convention alterations to the require for just-in-time fabricating and worldwide dispersion, the supply chain for investigational therapeutic items (IMPs) and clinical trial materials faces monstrous weight. In this environment, a apparently direct strategy—pooling—is developing as an undiscovered powerhouse for driving effectiveness, diminishing squander, and moderating risk.
Pooling, in the setting of clinical trials, alludes to the technique of bundling and labeling supplies in a adaptable way so they can be utilized over numerous conventions or ponders inside a clinical program, or maybe than being entirely committed to a single trial from the beginning. By deferring the point at which a supply unit is relegated to a particular study—sometimes right up to the minute of quiet dispensation—sponsors pick up unparalleled adaptability and considerable financial benefits.
Background and Verifiable Setting: From Settled Names to Adaptable Technology
The concept of resource-sharing in coordinations, for the most part, is not modern. Coordinations pooling has a long history in commercial supply chains (e.g., retail, fabricating) where competing or non-competing firms collaborate to share distribution centers, transportation, and dispersion systems to accomplish economies of scale and diminish natural impressions. Essentially, pooled acquirement components for pharmaceuticals and immunizations have existed for decades, outstandingly in worldwide wellbeing activities like the PAHO Rotating Support and the GCC, to total request, decrease unit costs, and progress get to to fundamental drugs in creating countries.
However, applying this to the specifics of clinical trial supplies, especially the IMPs themselves, is a more later evolution.
The Rise of Innovation as an Enabler
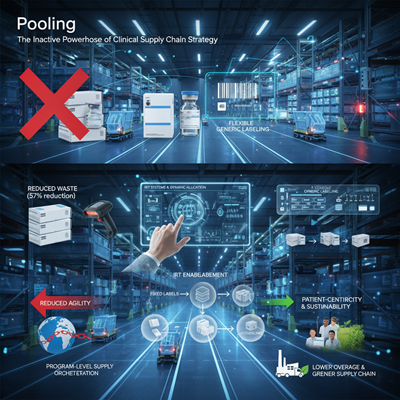
Historically, clinical supplies were burdened with pre-printed understanding numbers and particular visit identifiers specifically on the names, permanently devoting the sedate unit to a single understanding on a single convention. This hone was unbending and a essential source of tall squander (overage) when enrollment rates or think about plans changed.
The move started with the broad selection of Intuitively Reaction Innovation (IRT) frameworks, regularly alluded to as Randomization and Trial Supply Administration (RTSM) frameworks, roughly two decades ago.
“Not indeed 20 a long time prior, clinical supply fabric names included pre-printed understanding numbers and visit identifiers… As pooling advanced, the most common execution has been to have a stock of supplies at a terminal prepared and able to be devoted to one ponder or another up to the point of shipment to a site.”
IRT innovation permits the framework to oversee the randomization and supply allotment powerfully. This implied the physical name may be disentangled, or “genericized,” to incorporate as it were the essential center data, with the IRT framework carefully following and relegating the particular convention number and quiet points of interest at a afterward, more profitable point. This development was the key innovative enabler for advanced clinical supply pooling.
Current Patterns and Pooling Strategies
Today, clinical pooling is seen as a vital basic for any clinical program with different, related trials (e.g., a sedate being tried for a few signs or over diverse phases).
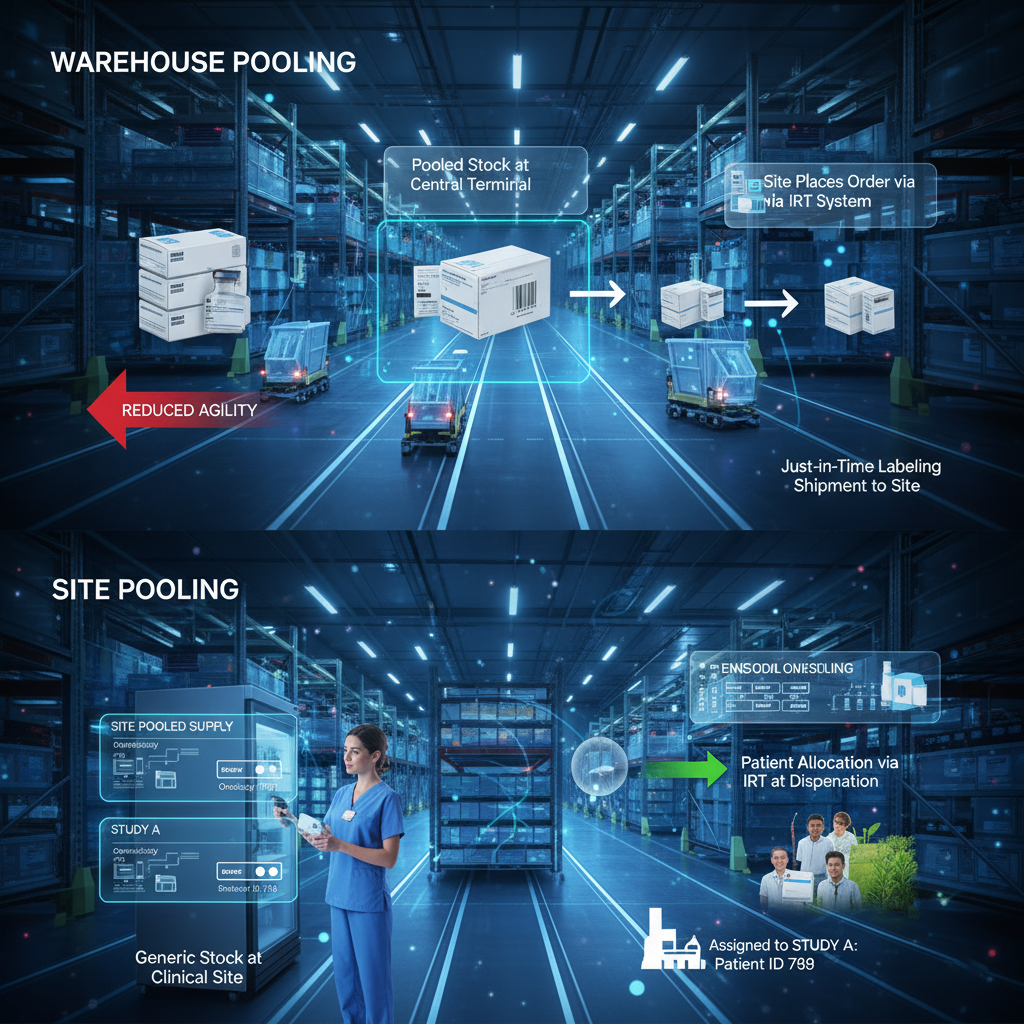
Types of Clinical Pooling
Pooling can be executed at distinctive stages in the supply chain, each advertising a unmistakable level of flexibility:
- Warehouse Pooling: The most common shape. Supplies are bundled and held at a central terminal (or numerous terminals) and are recorded in the IRT as being accessible for all thinks about in the pool. The minute a location places an arrange for a particular convention, the unit is at that point devoted to that convention, regularly through a Just-in-Time (JIT) labeling prepare at the terminal some time recently shipment.
- Site Pooling: A less common, but exceedingly adaptable, approach. Supplies are dispatched to a clinical location and stay pooled (i.e., usable for numerous trials at that location) until the minute of allotment to a quiet through the IRT framework. This offers greatest flexibility for locales overseeing a few trials utilizing the same compound, permitting supplies to flex to the trial with the quickest enrollment.
The Developing Require for Flexibility
Current clinical patterns heightening the require for pooling:
- Expanding Trial Complexity: Versatile trial plans, decentralized clinical trials (DCTs), and worldwide multi-site thinks about present characteristic enrollment instability, making precise determining about outlandish. Pooling acts as a fence against this uncertainty.
- Rise of Biologics and Progressed Treatments: Cell and quality treatments regularly include tall fabricating costs and rigid cold-chain prerequisites. Minimizing squander (overage) in these costly programs is critical.
- Quickened Improvement Timelines: The weight to move drugs through the pipeline quicker implies supply chains must be right away versatile to unused conventions being propelled or existing ones being amended.
Expert Suppositions and The Esteem Proposition
Experts over the clinical supply chain generally point to squander decrease, fetched reserve funds, and hazard moderation as the essential benefits of a strong pooling strategy.
The Financial and Operational Case
“The major preferences of the procedure stem from deferring the point in time in which a bundled unit is devoted to a ponder and in this manner maximizing the opportunity for the supplies to be utilized,” notes one industry VP.
- Diminished Overage and Squander: A common issue is a protocol’s overage (abundance supplies requested based on a tall figure). Instep of disposing of costly, devoted supplies, pooling permits them to be utilized by other trials in the program. This straightforwardly leads to critical IP fetched savings.
- Manufacturing Effectiveness: Pooling permits supports to arrange less, bigger, and more reliable fabricating and bundling parts. Bundling 10,000 bottles once is distant more productive than bundling 20 isolated parts of 500 bottles, each requiring line clearance, set-up, and quality testing.
- Mitigation of Stock-Out Chance: By making a central, adaptable buffer of stock, pooling guarantees progression of supply, diminishing the hazard of a “stock-out” in a quickly enlisting trial that seem something else lead to understanding measurements intrusions or misplaced enrollment opportunity.
Barriers to Broad Adoption
Despite the clear benefits, pooling has not come to its full potential, a estimation resounded by experts.
One critical specialized jump is the misalignment with conventional Endeavor Asset Arranging (ERP) frameworks. ERPs are planned to oversee completely characterized resources, meaning a sedate unit must be clearly related with a particular ponder for bookkeeping and following. Pooling, by its nature, requires the supply to exist in a “somewhat vague state” until assignment, which bequest ERP frameworks do not effortlessly support.
Other challenges include:
- Administrative Complexity: Actualizing pooling over different nations requires cautious route of differing import/export and labeling controls. The single part of pooled supplies must be affirmed for utilize in all conventions for which it is expecting. A delay in one protocol’s endorsement can end the utilize of the whole pooled parcel for all studies.
- System Customization: Whereas numerous IRT arrangements back pooling, it regularly requires critical customization or maybe than being a standard, out-of-the-box include, including to construct time and cost.
Implications for the Future of Clinical Development
For the pharmaceutical and biotech industry, pooling is not fair an operational strategy; it is a vital instrument that straightforwardly underpins the future of clinical development.

1. Empowering More noteworthy Program Deftness: As sedate improvement moves toward more complex, multi-arm, and versatile trials, a pooled stock framework is a need, not a extravagance. It gives the real-time adaptability required to quickly alter to approaching information, moving enrollment speeds, and new geographic needs.
2. Supporting Patient-Centricity: By guaranteeing progression of supply and decreasing the hazard of stock-outs, pooling supports a patient-centric demonstrate. It ensures that the right medicine is accessible when and where the quiet needs it, anticipating baffling and possibly destructive intrusions in treatment.
3. Driving Supportability and Obligation: In an time of expanded center on corporate social duty and natural supportability, minimizing the overage of costly, made, and regularly temperature-sensitive item is a major win. Less disposed of drugs and more solidified, effective shipments contribute straightforwardly to a greener supply chain.
The industry is at a urgent point. As trials gotten to be more complex and taken a toll weights mount, the long-term arrangement lies in moving past divided, study-by-study supply chains. The guarantee of Pooling is a future where clinical supply is overseen comprehensively at the program level, making a more productive, cost-effective, and eventually, more effective way to bringing modern treatments to market.





