The pharmaceutical industry’s commitment to quiet security pivots on the effectiveness and unwavering quality of its Worldwide Pharmacovigilance (GPV) frameworks. In an period of heightening information volumes and progressively exacting worldwide controls, the conventional show of on-premises GPV IT applications is getting to be a risk. The key relocation of these mission-critical frameworks to the cloud has developed as a essential, non-negotiable step for organizations looking for upgraded deftness, decreased costs, and the capacity to use transformative advances like Fake Insights (AI).
This in-depth, journalistic-style piece investigates the scene of GPV cloud movement, giving a speedy begin direct established in master supposition, verifiable setting, and current industry trends.
Background and Authentic Context
Pharmacovigilance systems—which track, survey, and anticipate antagonistic impacts from pharmaceutical products—have truly been housed in profoundly secure, on-premises information centers. This was generally driven by the foremost significance of information judgment, security, and administrative compliance with measures like GxP and FDA 21 CFR Portion 11.
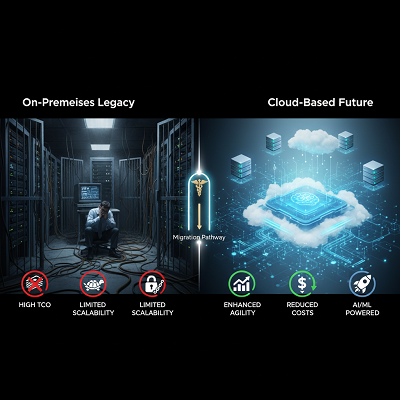
For decades, the on-premises demonstrate advertised a sense of control, but it came with noteworthy drawbacks:
- High Add up to Fetched of Proprietorship (TCO): Broad capital consumption on equipment, upkeep, and office overhead.
- Limited Adaptability: Trouble in quickly scaling assets to handle information spikes from clinical trials or post-market surveillance.
- Slow Advancement: Dull, expensive, and troublesome computer program updates to keep up administrative compliance, redirecting assets from innovation.
The move toward the cloud was at first reluctant, but the development of Cloud Benefit Suppliers (CSPs) like Amazon Web Administrations (AWS), Microsoft Sky blue, and Google Cloud Stage (GCP)—coupled with their strong security certifications and particular compliance offerings—has eased numerous of the beginning fears. The COVID-19 widespread served as a major quickening agent, driving pharmaceutical companies to embrace adaptable, cloud-based collaboration and information examination instruments to oversee pressing worldwide requests. Nowadays, the talk about is no longer if to move to the cloud, but how to do it successfully.
A Fast Begin Direct: Key Stages for GPV Cloud Migration
A fruitful cloud relocation for GPV applications is not fair an IT extend; it’s a commerce change. Specialists advocate for a organized, staged approach that prioritizes compliance and minimizes disturbance to persistent security workflows.
Phase 1: Appraisal and Technique (The ‘Why’ and ‘What’)
The to begin with step is setting up a comprehensive understanding of the current scene and characterizing clear objectives.
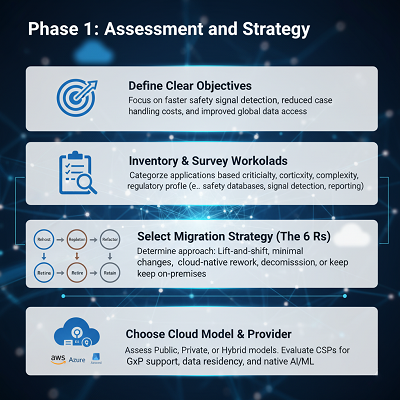
| Key Action | GPV-Specific Consideration |
| Define Clear Objectives to for disseminated teams. | Focus on results like quicker security flag location, decreased case handling costs, and progressed worldwide information get |
| Inventory and Survey Workloads and administrative profile. | Categorize GPV applications (e.g., security databases, flag location, administrative detailing) based on their criticality, complexity,and administrative profile. |
| Select Movement Methodology (The 6 Rs) (noteworthy rework for cloud-native), Held (cleared out on-premises), or Resigned (decommissioned). Profoundly customized, complex GPV frameworks regularly require Replatforming or Refactoring. | Determine if applications will be: Rehosted (lift-and-shift), Replatformed (negligible changes), Refactored/Rearchitected |
| Choose Cloud Demonstrate & Provider | Select a Open, Private, or Cross breed cloud demonstrate. Assess CSPs based on their compliance certifications (e.g., GxP back), information residency choices (pivotal for worldwide PV), and local AI/ML capabilities. |
Phase 2: Arranging and Plan (The ‘How’)
Detailed arranging guarantees the modern environment meets all compliance and execution benchmarks some time recently movement begins.
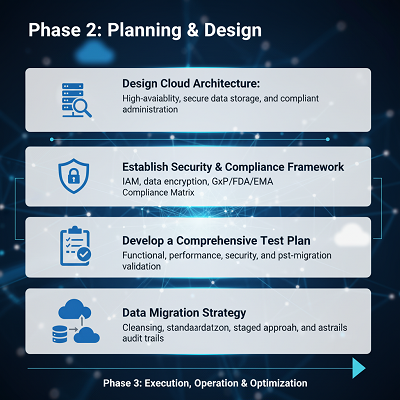
| Key Action | GPV-Specific Consideration |
| Design Cloud Architecture | Ensure the plan joins repetition and multi-availability zones for tall accessibility, minimizing downtime. Arrange for secure,compliant information administration and storage. |
| Establish Security & Compliance Framework | Implement vigorous Character and Get to Administration (IAM). Uphold information encryption at rest and in travel. Create a Compliance Lattice to outline all modern cloud forms to GxP, FDA, and EMA requirements. |
| Develop a Comprehensive Test Plan | This must incorporate utilitarian, execution, security, and compliance testing. A significant component is post-migration approval of trade forms to guarantee mechanized controls create the same, adjust outcomes. |
| Data Relocation Strategy | Plan for information cleansing, standardization, and change some time recently relocation. Select an approach (e.g., staged incremental exchange over a ‘big-bang’ exchange) to minimize operational disturbance. Keep up nitty gritty review trails of all information development to guarantee information integrity. |
Phase 3: Execution, Operation, and Optimization
Migration is frequently executed in reasonable, staged rollouts, frequently beginning with a littler, less-critical application to approve the approach (pilot program).
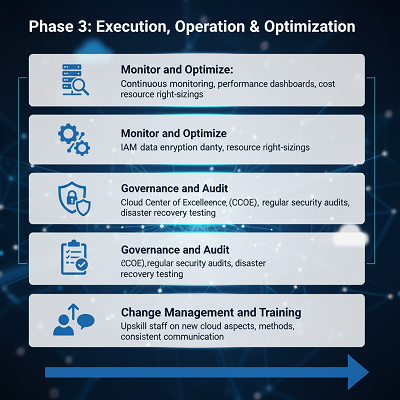
| Key Action | GPV-Specific Consideration |
| Monitor and Optimize | Implement nonstop checking of execution, accessibility, and taken a toll. Set up dashboards for key operational measurements (e.g., application reaction times). Right-size cloud assets to avoid taken a toll overruns. |
| Governance and Audit | Establish a Cloud Center of Greatness (CCoE). Routinely review the environment for progressing security and compliance. Test catastrophe recuperation and rollback procedures. |
| Change Administration and Training | Train and upskill pharmacovigilance and IT staff on modern cloud parts, get to strategies, and highlights. Clear and steady communication is crucial to overseeing organizational resistance. |
Current Patterns and Master Opinion
The development of GPV to the cloud is at the conversion of a few major innovative and administrative trends.
The AI/ML and Huge Information Imperative
The most critical driver is the require to use Manufactured Insights (AI) and Machine Learning (ML). Bequest, on-premises frameworks are regularly siloed and need the compute control to analyze the enormous, assorted datasets—including Real-World Prove (RWE) from electronic wellbeing records and social media—that are basic for proactive flag detection.
Expert See: “Moving to the cloud is key to quickening pharmacovigilance development,” states one industry whitepaper. “Cloud-based security applications have comprehensive APIs, effortlessly coordination with other data sources… empowering the most prominent esteem from their data.”
Trend: Cloud-based PV stages are overwhelming in the Pharmacovigilance Computerization Advertise, with the cloud-based fragment anticipated to support its lead. AI-powered devices are mechanizing manual exercises like case admissions and handling, altogether improving the speed and exactness of medicate security assessments.
Regulatory Arrangement and Compliance as a Service
CSPs are progressively giving Compliance-as-a-Service highlights, disentangling the burden of keeping up approval for pharmaceutical companies. Cloud situations can offer built-in security highlights, robotized security fixing, and comprehensive offerings that disentangle assembly worldwide administrative requirements.
Expert See: Cloud arrangements offer “consistent updates as unused usefulness gets to be accessible in a approved and compliant state,” tending to the exceedingly troublesome and costly nature of constrained updates in bequest on-premises frameworks taking after administrative changes.
Implication: This move permits pharmaceutical companies to divert IT assets from essential foundation support to high-value exercises like progressed information investigation and chance minimization strategies.
The Crossover Reality
While cloud selection is widespread—with the tremendous lion’s share of pharma companies utilizing cloud arrangements in a few form—few are 100% cloud-based. Numerous organizations keep up a crossover demonstrate, keeping a few profoundly basic or bequest applications on-premises whereas moving unused or less-sensitive workloads to the cloud. This offers a adjust of control, compliance, and adaptability, permitting for a slow, less-risky transition.

Implications for the Future of Pharmacovigilance
The fruitful relocation of GPV applications to the cloud has significant suggestions for worldwide persistent security and the pharmaceutical commerce model:
- Proactive Security: The marriage of cloud adaptability and AI/ML capabilities changes PV from a receptive announcing work to a proactive hazard distinguishing proof framework. Quicker preparing of antagonistic occasion reports and more modern flag location lead to speedier intercessions and more secure medicines.
- Global Harmonization: Cloud arrangements disentangle the handle of accomplishing worldwide administrative compliance and giving get to to a single, bound together security database for geologically scattered groups and Contract Investigate Organizations (CROs).
- Cost and Asset Proficiency: The operational proficiency of the cloud’s pay-as-you-go demonstrate and the diminishment in on-premises framework costs permit companies to contribute more in center logical R&D and basic medicate security functions.
- Talent and Abilities: The movement requires a move in required IT abilities, moving from overseeing physical servers to cloud administration, security, and stage optimization. Venture in upskilling inside groups is a basic suggestion for long-term success.
In conclusion, for worldwide pharmacovigilance, the cloud is more than an framework choice; it is the foundational stage that empowers the another era of medicate security and compliance. By taking after a fastidious, staged movement direct that keeps compliance, information astuteness, and commerce progression at its center, pharmaceutical companies can effectively explore the clouds and develop with a more spry, cost-effective, and eventually, more secure PV system.





